Published online Oct 6, 2018. doi: 10.12998/wjcc.v6.i11.441
Peer-review started: May 27, 2018
First decision: July 3, 2018
Revised: July 23, 2018
Accepted: August 19, 2018
Article in press: August 20, 2018
Published online: October 6, 2018
Processing time: 125 Days and 3.3 Hours
To investigate the diagnostic value of abnormal serum carbohydrate antigen 199 (CA199) level in acute cholangitis secondary to choledocholithiasis.
In this retrospective cohort study, the clinical data of 727 patients with choledocholithiasis admitted to the Third Affiliated Hospital of Zunyi Medical College from June 2011 to June 2017 were collected. Among these patients, 258 patients had secondary acute cholangitis and served as observation group, and the remaining 569 choledocholithiasis patients served as the control group. Serum liver function indexes and tumor markers were detected in both groups, and the receiver operating characteristic (ROC) curves were constructed for markers showing statistical significances. The cutoff value, sensitivity, and specificity of each marker were calculated according to the ROC curves.
The results of liver function tests showed no significant differences between the two groups (P > 0.05). Tumor markers including serum CA125, CA153, carcinoembryonic antigen, and alpha fetoprotein levels were also not significantly different (P > 0.05); however, the serum CA199 level was significantly higher in the observation group than in the control group (P < 0.05). The ROC curve analysis showed that the area under the curve was 0.885 (95%CI: 0.841-0.929) for CA199, and the cutoff value of 52.5 kU/L had the highest diagnostic accuracy, with a sensitivity of 86.8% and a specificity of 81.6%.
Abnormally elevated serum CA199 level has an important value in the diagnosis of acute cholangitis secondary to choledocholithiasis. It may be a specific inflammatory marker for acute cholangitis.
Core tip: Acute cholangitis is an acute inflammatory response to increased bile duct pressure and bacterial infection following biliary obstruction, whereas biliary obstruction is mostly caused by choledocholithiasis. Failure of timely predicting the onset of acute cholangitis may lose the chance for a minimally invasive surgery and result in a high mortality of the patients. In this study, a total of 727 choledocholithiasis patients were included, and the results suggest that abnormally elevated serum carbohydrate antigen 199 level has an important value in the diagnosis of acute cholangitis secondary to choledocholithiasis. It may be a specific inflammatory marker for acute cholangitis. As a convenient and rapid test, it is worthy to be applied in clinical settings.
- Citation: Mei Y, Chen L, Peng CJ, Wang J, Zeng PF, Wang GX, Li WP, Luo YQ, Du C, Liu K, Xiong K, Leng K, Feng CL, Jia JH. Diagnostic value of elevated serum carbohydrate antigen 199 level in acute cholangitis secondary to choledocholithiasis. World J Clin Cases 2018; 6(11): 441-446
- URL: https://www.wjgnet.com/2307-8960/full/v6/i11/441.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i11.441
Acute cholangitis is an acute inflammatory response to increased bile duct pressure and bacterial infection following biliary obstruction, whereas biliary obstruction is mostly caused by choledocholithiasis[1,2]. Bacteria and endotoxin in the infected biliary system can enter the blood circulation quickly, causing secondary systemic inflammatory reaction, septic shock, sepsis, and other serious complications, which threaten the life of the patients. Therefore, failure of timely predicting the onset of acute cholangitis may result in a high incidence of acute cholangitis and high mortality of the patients[3]. Furthermore, the patients may lose the chance for endoscopic retrograde cholangiopancreatography (ERCP), which is a mainstay of treatment for choledocholithiasis, instead, they have to undergo an emergency surgery that is more difficult, more risky, and associated with poor prognosis. In our clinical practice, some of the patients with acute cholangitis often had abnormally elevated tumor markers. To explore the values of these markers in the early diagnosis of acute cholangitis, we retrospectively analyzed the clinical data of acute cholangitis patients who were treated in our hospital in recent years.
In this retrospective cohort study, the clinical data of 727 choledocholithiasis patients were collected. Among them, 258 patients had secondary acute cholangitis and served as observation group, and the remaining 569 patients with choledocholithiasis alone served as the control group. Choledocholithiasis was suggested by B-ultrasound, computed tomography (CT), or magnetic resonance cholangiopancreatography before surgery and confirmed during surgery. Acute cholangitis secondary to choledocholithiasis was diagnosed if Charcot’s triad was found in these patients. The age, sex, diameter of common bile duct (CBD), number of CBD stones, complications and white blood cell (WBC) count were compared, with no significant differences between the two groups (P < 0.05) (Table 1).
| Observation group (n = 158) | Control group (n = 569) | χ2/t value | P value | |
| Age (yr) | 54.31 ± 12.54 | 51.65 ± 11.29 | 0.336 | 0.752 |
| Gender | ||||
| Male | 69 | 263 | 0.324 | 0.569 |
| Female | 89 | 306 | ||
| B-ultrasound | ||||
| Diameter of CBD (mm) | 9.51 ± 2.14 | 9.85 ± 2.07 | 0.243 | 0.851 |
| Number of CBD | ||||
| Single | 107 | 372 | 0.302 | 0.583 |
| Multiple | 51 | 197 | ||
| Complications | ||||
| Diabetes mellitus | 32 | 112 | 0.025 | 0.874 |
| Hypertensive disease | 36 | 101 | 2.049 | 0.152 |
| Hyperlipidemia | 13 | 41 | 0.188 | 0.665 |
| WBC count | 8.23 ± 1.82 | 8.68 ± 2.25 | -1.702 | 0.092 |
Diagnostic criteria for acute cholangitis[4] was still dependent on clinical manifestations and auxiliary examinations. (A) Clinical manifestations included: (1) history of biliary disease; (2) fever and/or chills; (3) jaundice; and (4) abdominal pain (right upper quadrant or epigastric). (B) Laboratory data included: evidence of inflammatory response and abnormal liver function tests. (C) Imaging findings included: biliary dilatation, or evidence of an etiology (stricture, stone or stent). Diagnosis could be established if the followings were found: (1) Charcot’s triad (2 + 3 + 4); (2) Two or more items in A + both items in B and item in C. The severity of acute cholangitis was divided into three grades: mild (grade I), moderate (grade II), and severe (grade III).
Inclusion criteria: Control group: with confirmed choledocholithiasis; with typical manifestations of obstructive jaundice [total bilirubin (TBiL) > 17.1 μmol/L; increased direct bilirubin (DBil) level; DBiL/TBiL ratio > 50%)]; and without manifestation of acute biliary tract inflammation (including acute cholecystitis or cholangitis). Observation group: with confirmed choledocholithiasis; with the presence of acute cholangitis (grade I or grade II); and without severe biliary tract infection including acute suppurative cholecystitis or acute severe cholangitis (grade III).
Exclusion criteria: (1) Accompanied with intrahepatic bile duct stones; (2) accompanied with malignant jaundice disease, including cholangiocarcinoma, gallbladder cancer, liver cancer, pancreatic head cancer, and/or ampulla carcinoma; (3) accompanied with acute pancreatitis, active hepatitis, and/or liver cirrhosis; and (4) accompanied with other systemic inflammatory diseases.
The baseline clinical data of both groups were analyzed. Serum liver function indicators aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase, alkaline phosphatase (ALP), total TBil, DBil, and tumor markers carbohydrate antigen 199 (CA199), CA125, CA153, carcinoembryonic antigen (CEA), and alpha fetoprotein (AFP) were detected in both groups. Receiver operating characteristic (ROC) curves were constructed for markers showing significant differences between the two groups. The cutoff value, sensitivity, and specificity of each marker were calculated according to the ROC curves.
Results of serum liver function indicators and tumor markers were compared between the two groups. The ROC curves were used to analyze the results.
The serum liver function indicators, including ALT, AST, TBil, DBil, glutamyl transpeptidase, and ALP, showed no significant differences between the observation group and the control group (P > 0.05) (Table 2).
| Observation group (n = 158) | Control group (n = 569) | t value | P value | |
| ALT | 127.51 ± 28.71 | 125.34 ± 27.25 | 0.235 | 0.867 |
| AST | 130.29 ± 27.87 | 132.37 ± 30.19 | 0.229 | 0.871 |
| TBiL | 101.06 ± 23.38 | 95.49 ± 22.42 | 0.824 | 0.206 |
| DBiL | 60.62 ± 11.73 | 55.31 ± 10.82 | 0.926 | 0.179 |
| GGT | 210.28 ± 43.75 | 186.35 ± 41.08 | 1.083 | 0.126 |
| ALP | 237.84 ± 52.97 | 218.47 ± 50.18 | 1.024 | 0.145 |
The serum CA199 level was significantly higher in the observation group than in the control group (P < 0.05). In contrast, serum levels of CA125, CA153, CEA, and AFP were all not significantly different (P > 0.05) (Table 3).
| Observation group (n = 158) | Control group (n = 569) | t value | P value | |
| CA199 | 90.25 ± 21.17 | 25.19 ± 7.06 | 25.597 | 0.000 |
| CA125 | 26.73 ± 6.94 | 24.61 ± 6.45 | 0.527 | 0.487 |
| CA153 | 10.07 ± 2.54 | 9.76 ± 2.24 | 0.353 | 0.728 |
| CEA | 2.67 ± 0.57 | 2.53 ± 0.15 | 0.301 | 0.779 |
| AFP | 3.18 ± 0.63 | 3.12 ± 0.61 | 0.208 | 0.894 |
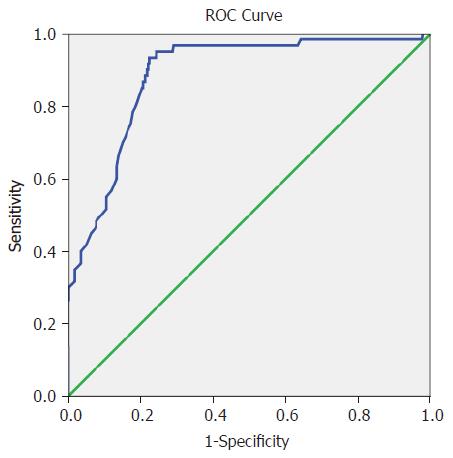
The area under the curve (AUC) was 0.885 (95%CI: 0.841-0.929) for CA199, and the cutoff value of 52.5 kU/L had the highest diagnostic accuracy, with a sensitivity of 86.8% and a specificity of 81.6% (Table 4 and Figure 1).
| Area | Std. error | Asymptotic sig. | Asymptotic 95%CI | Cutoff value | Sensitivity | Specificity |
| 0.885 | 0.022 | 0.000 | 0.841-0.929 | 52.5 | 0.868 | 0.816 |
Choledocholithiasis is a common and frequently occurring disease in departments of surgery. Its incidence is particularly high in Southwest China and has been rising in recent years. The main treatments for choledocholithiasis include open or laparoscopic cholecystectomy and ERCP combined with endoscopic sphincterotomy[5-7]. Persistent presence of stones in the CBD may affect bile excretion, often leading to development of secondary acute cholangitis. Although there are various methods to diagnose CBD stones and examination techniques have been increasingly improved, there is no significant decline in the incidence, severity, and mortality of acute cholangitis secondary to choledocholithiasis. The main reasons are the lack of objective evaluation markers and methods for the early diagnosis of acute cholangitis. Charcot’s triad has long been used as a specific manifestation of acute cholangitis; however, its clinical practicality and reliability is questionable due to its low sensitivity (about 26%)[3], especially in elderly inpatients. Delayed or improper treatment can worsen the condition of the patients[8-9]. Therefore, it is of great clinical significance to identify the early predictors of acute cholangitis secondary to choledocholithiasis so as to develop rational surgical protocol avoid postoperative complications and reduce mortality.
According to the Tokyo criteria of acute cholangitis, it can be diagnosed if the clinical manifestations of Charcot’s triad, i.e., fever and/or chills, abdominal pain (right upper quadrant or epigastric), and jaundice are present[4]. Charcot’s triad is the main clinical manifestation, whereas the auxiliary examinations mainly include the measurements of common inflammatory markers and the imaging of biliary obstruction. The commonly used inflammatory indicators include blood WBC count, neutrophil ratio, serum C-reactive protein, and procalcitonin. Imaging examinations for biliary obstruction included ultrasound, CT, MRI, endoscopic ultrasonography, and cholangiography. Unfortunately, up to date, well-recognized early predictors of acute cholangitis secondary to choledocholithiasis have been largely unavailable. CA199 is a mucin-like substance usually embedded on the surface of epithelial cells in the bile duct, pancreatic duct, stomach, and prostate. The serum CA199 level is low in normal subjects[10]. Numerous studies have demonstrated that the biological function of CA199 is mainly involved in cell proliferation, differentiation, signal transduction, apoptosis, and immune regulation. In particular, CA199 can promote leukocyte aggregation by regulating leukocyte migration and adhesion in inflammatory regions[11]. As one of the common tumor markers, CA199 is mainly used in the auxiliary diagnosis and evaluation of disease progression and prognosis of pancreatic cancer, colorectal cancer, and other diseases[12-13]. In recent years, the abnormally elevated CA199 in some non-cancerous benign diseases has drawn increasing attention[14-16]. However, the role of elevated serum CA199 in the diagnosis of acute cholangitis secondary to choledocholithiasis deserves further studies.
In the present study, there was no statistically significant difference in the serum liver function indicators between the observation group and control group, suggesting that the changes of serum liver function indicators do not necessarily imply the occurrence of secondary acute cholangitis in patients with obstructive jaundice due to biliary duct stones. Comparisons of tumor markers showed that the levels of CA125, CA153, CEA, and AFP were not significantly different between the two groups, suggesting that the levels of these tumor markers were not directly related to the occurrence of acute cholangitis. However, our study found that the serum CA199 level in the observation group was significantly higher than in the control group, indicating that the abnormal elevation of serum CA199 level may have potential predictive value for the occurrence of acute cholangitis following choledocholithiasis, i.e., CA199 may be a special inflammatory marker for the onset of acute cholangitis. The ROC curve analysis showed that the AUC was relatively large for CA199, and the cutoff value of 52.5 kU/L had the highest sensitivity and specificity. It is suggested that clinicians must deal with the disease early, avoid the occurrence of acute cholangitis, relieve the pain of patients, and improve the prognosis of the disease. The elevation of serum CA199 level in acute cholangitis patients may be related with the fact that inflammation stimulates the proliferation of bile duct epithelial cells, resulting in the increased secretion of CA199 and other inflammatory mediators. In addition, the accumulation of a large amount of CA199 in the bile duct, and the obstructed bile duct increase the bile duct pressure, leading to the backflow of CA199 into blood stream. Meanwhile, thickening of the bile duct wall during acute cholangitis lowers the ability of the bile duct in scavenging CA199 and other substances, leading to persistent high pressure in the bile duct, even destroying the peripheral vascular mucosal barrier, thus promoting backflow of CA199 into the bile duct.
In summary, abnormally elevated serum CA199 level may be a potentially useful marker for the early prediction of acute cholangitis secondary to choledocholithiasis. When the CA199 level reaches the cutoff value, physicians should be vigilant about the possibility of acute cholangitis, and timely and proper interventions should be carried out to avoid aggravation of the disease.
Choledocholithiasis is a common and frequently occurring disease in departments of surgery. Persistent presence of stones in the common bile duct may affect bile excretion, often leading to development of secondary acute cholangitis. Failure of timely predicting the onset of acute cholangitis may lose the chance for a minimally invasive surgery and result in a high mortality of the patients.
It is of great clinical significance to explore the methods for the early diagnosis of acute cholangitis so as to develop rational surgical protocol, avoid postoperative complications and reduce mortality.
To investigate the diagnostic value of abnormal serum carbohydrate antigen 199 (CA199) level in acute cholangitis secondary to choledocholithiasis.
In this retrospective cohort study, the clinical data of 727 patients with choledocholithiasis were collected. Serum liver function indexes and tumor markers were detected in both groups, and the ROC curves were constructed for markers showing statistical significances.
The serum CA199 level was significantly higher in the observation group than in the control group (P < 0.05). The receiver operating characteristic curve analysis showed that the area under the curve was 0.885 (95%CI: 0.841-0.929) for CA199, and the cutoff value of 52.5 kU/L had the highest diagnostic accuracy, with a sensitivity of 86.8% and a specificity of 81.6%.
Abnormally elevated serum CA199 level has an important value in the diagnosis of acute cholangitis secondary to choledocholithiasis.
Abnormally elevated serum CA199 level may be a potentially useful marker for the early prediction of acute cholangitis secondary to choledocholithiasis.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Touil-Boukoffa C, Kitamura K, Mercado M, Ahmed M S- Editor: Dou Y L- Editor: Filipodia E- Editor: Song H
| 1. | Yamamiya A, Kitamura K, Ishii Y, Mitsui Y, Nomoto T, Yoshida H. Feasibility of initial endoscopic common bile duct stone removal in patients with acute cholangitis. World J Clin Cases. 2017;5:280-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Tomizawa M, Shinozaki F, Hasegawa R, Shirai Y, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N. Comparison of acute cholangitis with or without common bile duct dilatation. Exp Ther Med. 2017;13:3497-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 424] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 4. | Wada K, Takada T, Kawarada Y, Nimura Y, Miura F, Yoshida M, Mayumi T, Strasberg S, Pitt HA, Gadacz TR. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:52-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 5. | Baucom RB, Feurer ID, Shelton JS, Kummerow K, Holzman MD, Poulose BK. Surgeons, ERCP, and laparoscopic common bile duct exploration: do we need a standard approach for common bile duct stones? Surg Endosc. 2016;30:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Huang Y, Feng Q, Wang K, Xiong X, Zou S. The safety and feasibility of laparoscopic common bile duct exploration for treatment patients with previous abdominal surgery. Sci Rep. 2017;7:15372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Liu Z, Zhang L, Liu Y, Gu Y, Sun T. Efficiency and Safety of One-Step Procedure Combined Laparoscopic Cholecystectomy and Eretrograde Cholangiopancreatography for Treatment of Cholecysto-Choledocholithiasis: A Randomized Controlled Trial. Am Surg. 2017;83:1263-1267. [PubMed] |
| 8. | Lee F, Ohanian E, Rheem J, Laine L, Che K, Kim JJ. Delayed endoscopic retrograde cholangiopancreatography is associated with persistent organ failure in hospitalised patients with acute cholangitis. Aliment Pharmacol Ther. 2015;42:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Isayama H, Yasuda I, Tan D. Current strategies for endoscopic management of acute cholangitis. Dig Endosc. 2017;29 Suppl 2:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Scarà S, Bottoni P, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (1)] |
| 11. | Goh SK, Gold G, Christophi C, Muralidharan V. Serum carbohydrate antigen 19-9 in pancreatic adenocarcinoma: a mini review for surgeons. ANZ J Surg. 2017;87:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Yu Z, Chen Z, Wu J, Li Z, Wu Y. Prognostic value of pretreatment serum carbohydrate antigen 19-9 level in patients with colorectal cancer: A meta-analysis. PLoS One. 2017;12:e0188139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Luo G, Jin K, Cheng H, Liu C, Guo M, Lu Y, Yang C, Xu J, Wang W, Gao H. Carbohydrate antigen 19-9 as a prognostic biomarker in pancreatic neuroendocrine tumors. Oncol Lett. 2017;14:6795-6800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Amini E, Pishgar F, Hojjat A, Soleimani M, Asgari MA, Kajbafzadeh AM. The role of serum and urinary carbohydrate antigen 19-9 in predicting renal injury associated with ureteral stone. Ren Fail. 2016;38:1626-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Fukasawa H, Kaneko M, Niwa H, Yasuda H, Kumagai H, Furuya R. Carbohydrate antigen 19-9 is significantly elevated in autosomal dominant polycystic kidney disease. Nephrology (Carlton). 2018;23:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kajbafzadeh AM, Keihani S, Kameli SM, Hojjat A. Maternal Urinary Carbohydrate Antigen 19-9 as a Novel Biomarker for Evaluating Fetal Hydronephrosis: A Pilot Study. Urology. 2017;101:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |