Published online Jan 16, 2018. doi: 10.12998/wjcc.v6.i1.6
Peer-review started: November 17, 2017
First decision: November 30, 2017
Revised: December 6, 2017
Accepted: December 14, 2017
Article in press: December 15, 2017
Published online: January 16, 2018
Processing time: 55 Days and 20.8 Hours
Occlusion of the common carotid artery (CCA) is rare. CCA occlusion (CCAO) can present as drowsiness and right hemiplegia related to emboli after total arch replacement. Although we selected a follow-up at first because color duplex sonography showed retrograde flow from the left external carotid artery to the internal carotid artery, this patient had epilepsy and single-photon emission computed tomography (SPECT) acquired quantitative results of actual brain perfusion and showed insufficient collateral blood flow. To improve brain perfusion, we performed a bypass of the left subclavian artery to left CCA bypass. Postoperatively, the patient did not have epilepsy and drowsiness. Also, right hemiplegia improved enough for him to walk with support. SPECT showed increased left cerebral flow (the asymmetry ratio was 71% to 81%). Evaluation of the carotid artery with color duplex sonography alone was insufficient when CCAO showed retrograde or collateral flow. We should have performed quantitative evaluation with SPECT at the same time.
Core tip: Common carotid artery occlusion (CCAO) can include neurologic symptoms caused by low cerebral perfusion; however, blood flow in the internal carotid artery and external carotid artery is maintained by collateral circulation in most cases. In the former, we can noninvasively estimate the presence and intensity of collateral flow by single-photon emission computed tomography. In the latter, color flow duplex examination detects the patency of the distal vessels. Patients with CCAO should undergo estimation of the patency of their distal CCA and cerebral perfusion at the same time. Surgical management requires safe and effective strategies for symptomatic CCAO.
- Citation: Matsuda Y, Koyama T. Evaluation of revascularization after total arch replacement in common carotid artery occlusion. World J Clin Cases 2018; 6(1): 6-10
- URL: https://www.wjgnet.com/2307-8960/full/v6/i1/6.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i1.6
Occlusion of the common carotid artery (CCA) is rare. It occurs in 2%-4% of patients undergoing angiography for symptomatic cerebrovascular disease[1]. The natural history of the disease is unknown; therefore, certain parameters such as distal vessel patency and the presence of symptoms may affect treatment decisions[1].
CCA occlusion (CCAO) can include neurologic symptoms caused by low cerebral perfusion or emboli from the carotid stump; however, blood flow in the internal carotid artery (ICA) and external carotid artery (ECA) is maintained by collateral circulation in most cases[2]. In the former, we can noninvasively estimate the presence and intensity of collateral flow in patients with cerebrovascular disease by arterial spin-labeling magnetic resonance imaging or single-photon emission computed tomography (SPECT)[3,4]. In the latter, color flow duplex examination detects emboli and the patency of the distal vessels. Recognizing the patency of the distal vessels is important because it may allow for effective surgical revascularization when treating CCAO[5].
We report the importance of carotid artery flow and actual brain perfusion evaluated simultaneously in a patient with CCAO who underwent total arch replacement (TAR).
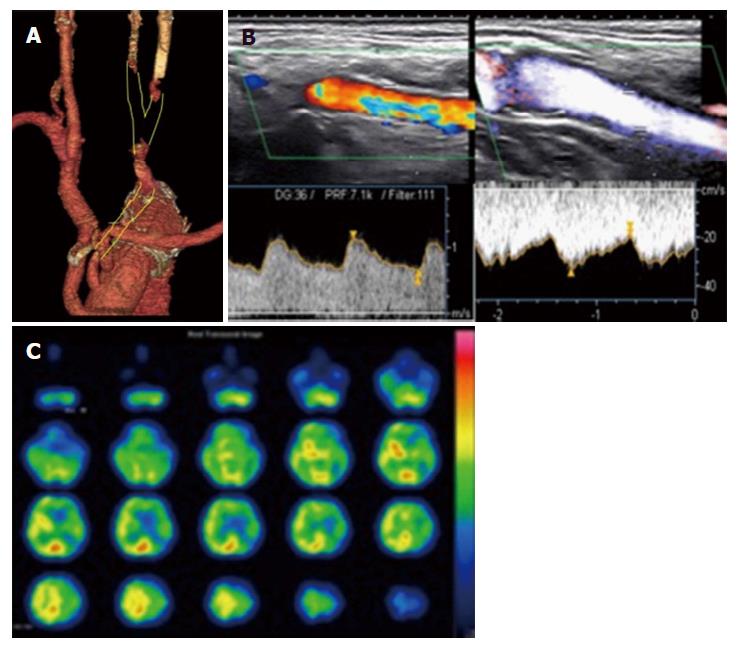
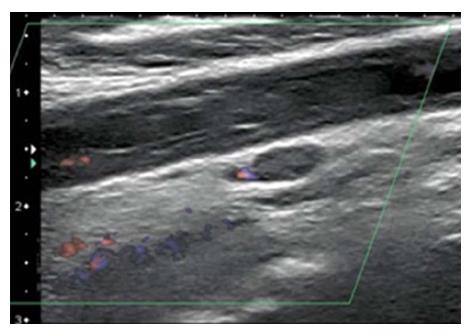
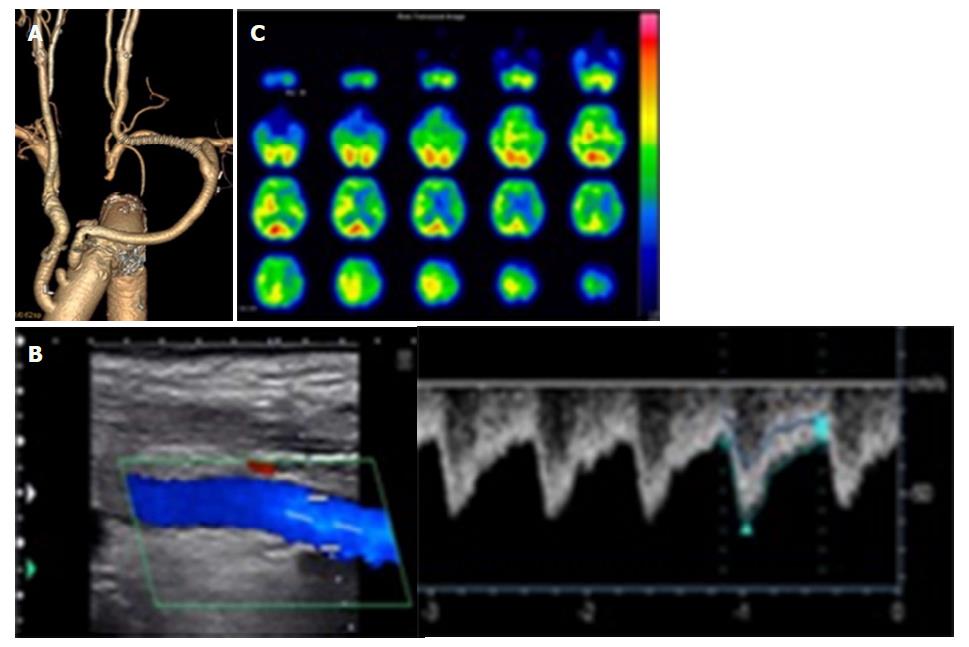
An 80-year-old man with hypertension who reported abdominal distention underwent computed tomography (CT), which detected a cystic thoracic aneurysm of the arch by chance. We performed TAR (Triplex 26 mm with 4 branches + Triplex 8 mm) with a frozen elephant trunk (J-graft 33 mm × 6 mm). At 3 h after surgery, right hemiplegia was present and CT showed that the left basal ganglia had a low-density area. On day 1, enhanced CT showed occlusion of the left CCA from the anastomotic site to near the bifurcation of the ECA and ICA (Figure 1A). On day 4, color duplex sonography showed occlusion of the left CCA related to emboli and retrograde flow from the left ECA to the ICA; the flow of the ICA decreased compared to that of the ECA (Figure 1B). Despite gradual improvement of his neurological symptoms, he had epilepsy on day 9. When we performed SPECT, his left cerebral flow was low (Figure 1C). In addition, color duplex sonography showed a floating thrombus of the distal CCA (Figure 2). To increase the left cerebral flow and remove the thrombus related to stroke, he underwent bypass of the left subclavian artery to the left CCA (FUSION Vascular Graft 6 mm; Maquet Cardiovascular, Wayne, NJ, United States). The distal anastomosis maintained bifurcation flow of the ECA and ICA (Figure 3A). Because the occluded part of the CCA was organized, we could only partially remove the floating thrombus. Postoperatively, the patient did not have epilepsy and right hemiplegia improved enough for him to walk with support. Color duplex sonography showed increased ICA flow (Figure 3B) and SPECT showed increased left cerebral flow (Figure 3C). The patient was transferred to another hospital 55 d after the first surgery to undergo rehabilitation.
Clinical features of ICA occlusion have been established; however, those of CCAO have not been established because it is rare. Using carotid sonography for 5400 patients with carotid arterial disease, the frequency of CCA occlusion was found to be 0.24% and that of ICA occlusion was found to be 2.5%[6]. Currently, studies regarding CCAO are available, but its management has not been established. We performed therapy based on CCAO without postoperative information because there is no coherent report of complications after TAR and there is no recommended method of treatment.
The natural history of CCAO is limited and may be associated with stroke, transient ischemic attack, or chronic cerebral ischemia. Cerebral dysfunction with ischemia may be connected to cytokines, prostanoids, and nitric oxide release[7]. In addition, the majority (92.7%) of patients treated were symptomatic[1]. Although most patients with patent bifurcation presented with amaurosis fugax and vertigo attacks, no patients with patent distal vessels and well-functioning intracranial collaterals had a major stroke. They had what was defined as a combination of a disturbance in consciousness and at least two neurological signs (conjugate deviation, homonymous hemianopia, aphasia, and hemiplegia)[5]. In our case, hemiplegia was caused by a major stroke, which was associated with plaque of the shaggy aorta perioperatively. Plaque occluded in the CCA was organized, and part of the thrombus in the organized plaque was floating postoperatively. Although we selected a follow-up at first because there is retrograde flow from the ECA to the ICA, we had to remove the floating emboli because it created a risk for worsening symptoms.
Recently, Parthenis et al[8] categorized CCAO into five types: Type Ia, which involves patent distal vessels (from the ECA to the ICA); type Ib, which involves patent distal vessels (from the ICA to the ECA); type II, which involves a patent ECA only; type III, which involves a patent ICA; and type IV, which involves an occluded ICA and ECA. In this study, most cases could be categorized as type Ia or type IV[8]. Perfusion of the ipsilateral cerebral hemisphere is provided by the collateral circulation: the ipsilateral ECA in retrograde, the ipsilateral subclavian artery, the contralateral ECA, and the circle of Willis intracranially[8,9]. Angiography is often performed to diagnose these types. However, we were not able to obtain detailed information such as collateral filling and flow[1]. Color duplex sonography is a sensitive method for estimating the dynamic pattern of collateral flow to reconstitute a patent carotid bifurcation and is the hallmark for detecting a patent ICA despite CCAO[5,10]. In our case, retrograde flow was maintained in the ECA and in the ICA. However, SPECT, which was used to perform the quantitative evaluation of actual brain perfusion, showed collateral blood flow insufficiency. Even if collateral flow is maintained, patients with neurological symptoms need to undergo quantitative evaluation of perfusion in the brain because there may be insufficiency[1].
Indications for surgical treatments are ipsilateral transient ischemic attack, recent nondisabling hemispheric stroke, and transient nonhemispheric cerebral symptoms or prophylactic revascularization before major surgical interventions[11]. The aim of surgical treatment is to therapeutically improve brain circulation and functionality in patients with cerebral insufficiency due to CCAO. Klonaris et al[1] reported that all patients undergoing surgery for transient nonhemispheric symptoms due to circulatory insufficiency experienced resolution of their symptoms after the procedure. Although we observed improved neurological symptoms without SPECT, we should aggressively estimate and treat low cerebral flow when it is related to neurological symptoms. Successful revascularization is dependent on precisely determining the presence and adequacy of collateral blood flow, thereby establishing patency of the distal CCA and acquiring quantitative evaluation results of actual brain perfusion[5].
In addition to accomplishing a successful procedure, it was necessary to not allow the floating emboli into the intracranial vessels. In this case, the distal CCA clamp during the bypass procedure was effective for preventing stroke and maintaining intracranial flow perioperatively.
In conclusion, patients with CCAO who continue to have neurological symptoms should be assessed both the blood flow and the brain perfusion.
An 80-year-old man with a cystic thoracic aneurysm of the arch was performed total arch replacement.
Drowsiness and right hemiplegia related to emboli after total arch replacement.
Embolism, thrombosis, aorta dissection, infection.
Single-photon emission computed tomography showed the left cerebral flow was low.
The authors performed bypass of the left subclavian artery to the left common carotid artery (CCA).
Martin RS 3rd reported indications for surgical treatments for CCA occlusion.
Patients with CCA occlusion who continue to have neurological symptoms should be assessed both the blood flow and the brain perfusion.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Anis S, Kvolik S, Sergi CM, Sijens PE S- Editor: Ji FF L- Editor: A E- Editor: Song XX
| 1. | Klonaris C, Kouvelos GN, Kafeza M, Koutsoumpelis A, Katsargyris A, Tsigris C. Common carotid artery occlusion treatment: revealing a gap in the current guidelines. Eur J Vasc Endovasc Surg. 2013;46:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Hass WK, Fields WS, North RR, Kircheff II, Chase NE, Bauer RB. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA. 1968;203:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 283] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling MRI can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011;42:2485-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Otomo K, Endo H, Ishikura K, Kodama S, Sato N, Fujiwara S. 3D arterial spin labeling for hemodynamic assessment after carotid artery revascularization: comparison with SPECT (in Japanese). Rinsyouhousyasen. 2015;60:1585-1596. |
| 5. | Bajkó Z, Bălaşa R, Moţăţăianu A, Maier S, Chebuţ OC, Szatmári S. Common carotid artery occlusion: a case series. ISRN Neurol. 2013;2013:198595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Nakamura A, Wakugawa Y, Yasaka M, Ogata T, Yasumori K, Kitazono T, Okada Y. Antegrade internal carotid artery collateral flow and cerebral blood flow in patients with common carotid artery occlusion. J Ultrasound Med. 2012;31:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Sergi C, Shen F, Lim DW, Liu W, Zhang M, Chiu B, Anand V, Sun Z. Cardiovascular dysfunction in sepsis at the dawn of emerging mediators. Biomed Pharmacother. 2017;95:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Parthenis DG, Kardoulas DG, Ioannou CV, Antoniadis PN, Kafetzakis A, Angelidou KI, Katsamouris AN. Total occlusion of the common carotid artery: a modified classification and its relation to clinical status. Ultrasound Med Biol. 2008;34:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Tsai CF, Jeng JS, Lu CJ, Yip PK. Clinical and ultrasonographic manifestations in major causes of common carotid artery occlusion. J Neuroimaging. 2005;15:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Dermitzakis I, Minardos I, Kampanarou M, Mitakou D. Color duplex sonography of occlusion of the common carotid artery with reversed flow in the extracranial internal carotid artery. J Clin Ultrasound. 2002;30:388-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Martin RS 3rd, Edwards WH, Mulherin JL Jr, Edwards WH Jr. Surgical treatment of common carotid artery occlusion. Am J Surg. 1993;165:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |