Published online Sep 16, 2017. doi: 10.12998/wjcc.v5.i9.368
Peer-review started: January 6, 2017
First decision: March 7, 2017
Revised: April 20, 2017
Accepted: June 30, 2017
Article in press: July 3, 2017
Published online: September 16, 2017
Processing time: 257 Days and 7.1 Hours
We report an atypical case of anti-N-methyl-D-aspartate receptor encephalitis (ANMDARE). A 27-year-old man diagnosed with ANMDARE received immunotherapy and had a good recovery. However, within one month, he developed severe status epilepticus and decreased level of conscience with new hyperpyrexia and dyspnea, and was admitted to the emergency intensive care unit. Acinetobacter baumanii were found in the sputum culture; and anti-NMDAR antibodies were positive (titer: 1/80) in the cerebrospinal fluid. Repeated immunotherapy was administered with antibacterial agents, and the patient recovered except for mild psychiatric sequelae. This is the first report of ANMDARE that aggravates after acinetobacter baumannii pneumonia. Awareness and knowledge of this disorder should be extended, especially in the emergency medicine community.
Core tip: In this paper we presented a very rare case of an anti-N-methyl-D-aspartate receptor encephalitis in which the patient aggravated after acinetobacter baumannii pneumonia and well responded to immunotherapy. The mechanism underlying the association needs attention.
- Citation: Wang CC, Li DJ, Xia YQ, Liu K. Anti-N-methyl-D-aspartate receptor encephalitis that aggravates after acinetobacter baumannii pneumonia: A case report. World J Clin Cases 2017; 5(9): 368-372
- URL: https://www.wjgnet.com/2307-8960/full/v5/i9/368.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i9.368
Anti-N-methyl-D-aspartate receptor encephalitis (ANMDARE) was initially identified in young females with ovarian tumor and was classified as a new immune-mediated encephalitis, producing antibodies to the target ANMDARE[1]. Following a growing attention paid to this disease, an increasing number of reports and analyses have appeared in recent years[2-5]. Early in the disease course, ANMDARE may resemble the first presentation of viral encephalitis[2]. Compared with viral encephalitis, clinical features including psychosis, hallucinations, behavior and personality changes, seizure activity were observed more often among cases of ANMDARE[2]. This disorder is confirmed by the identification of NMDAR antibodies in cerebrospinal fluid (CSF) or serum[1,2]. It deteriorates rapidly with no or delayed treatment, but commonly improves with the application of immunotherapy or through a tumor resection, if applicable. The recovery process is slow, taking nearly 18 mo[3]. Complications in patients while receiving treatment are yet to be established. Acinetobacter baumannii pneumonia has rarely been described in patients with ANMDARE[4,6]. We here report a case of ANMDARE in a man whose clinical condition had improved after immunotherapy, but became worse in no more than one month due to acinetobacter baumannii pneumonia.
A 27-year-old man without a previous medical history, initially presenting with an acute psychiatric syndrome, including personality and behavioral change, was first evaluated by a psychiatrist and admitted to the psychiatric department in our hospital. He behaved friendly with focused attention and clear consciousness, but responded slowly to questions and manifested with emotional instability. Interruption of thinking, and thinking burst were observed in psychiatric examination with absence of hallucination, delusion and impulsive aggression. Psychotropic drugs were used, but nothing worked. Rigidity emerged in seven days after admission. On day 12, he was transferred to neurology department with suspected viral encephalitis, in consideration of neurological impairment, including abnormal movements and tachycardia. He soon progressed to status epilepticus, insomnia, confusion of consciousness, and memory deficits, but without fever.
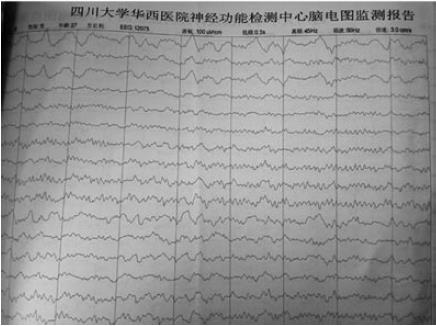
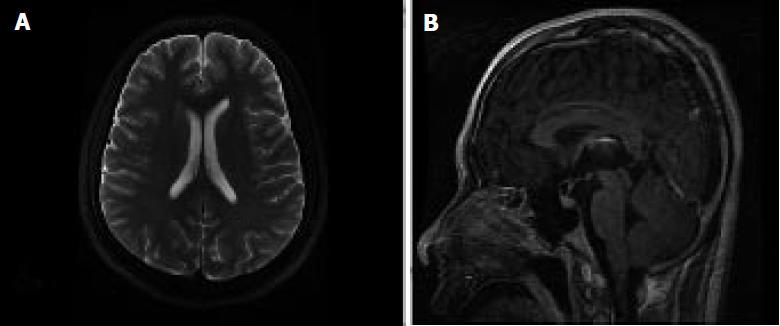
An electroencephalogram (EEG) showed diffuse or general slowing in the patient (Figure 1). Magnetic resonance imaging (MRI) of the brain revealed no abnormal changes (Figure 2). The first evaluation of CSF in psychotropic department was analyzed with ignorance of antibody detection. It only showed mild elevations of the protein level (90 mg/dL) and karyocyte count (50 × 106/L), with a normal glucose level (Table 1). In the neurology department, antibody against NR1 heteromeric NMDAR was detected in the CSF (titer: 1/100), and the serum (titer: 1/10). Comprehensive tumor screening, including repeated enhanced computerized tomography (CT) and a urogenital examination for testicular tumors, was performed, with no tumors or inflammatory lesions detected. The patient was eventually diagnosed with ANMDARE without tumors, and was immediately treated with anti-epileptic drugs, corticosteroids (steroid pulse therapy of methylprednisolone 1 g/d for 5 d, followed by tapered-off oral prednisolone) as well as intravenous immunoglobulin (IVIG, 400 mg/kg daily for 5 d). After these treatments, his general condition gradually improved and he was discharged from hospital, with clear mind and normal behaviors.
| First hospitalization | Post-treatment | Secondhospitalization | |
| Cerebrospinal pressure | Normal | Normal | Normal |
| NMDAR-Ab | 1:100(+) | - | 1:80(+) |
| CSF protein | 0.9 | 0.27 | 0.8 |
| (g/L) | |||
| CSF glucose | 4.26 | 3.88 | 3.66 |
| (mmol/L) | |||
| CSF chlorine | 12.6 | 128 | 120.1 |
| (mmol/L) | |||
| CSF karyota count | 50 × 106/L | 0 | 60 × 106/L |
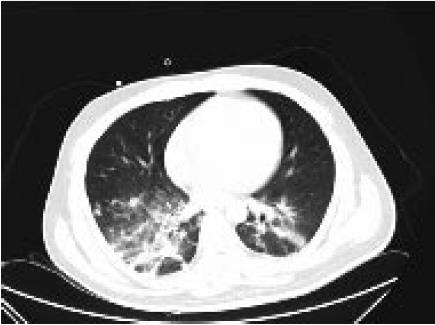
However, no more than one month after the initial onset, the man typically manifested with new hyperpyrexia and dyspnea and then developed severe status epilepticus, decreased level of consciousness and convulsion of the limbs again. He was admitted to the emergency intensive care unit (EICU) where supplemental oxygen via a nonrebreather mask was initiated. The temperature was up to 39.0 °C. On physical examination, perspiration and cutaneous pallor were observed. He presented mild tachypnea (35 bpm) and severe hypoxemia (SaO2 86%). On auscultation, he had a good air entrance bilaterally with scattered to diffuse crackles and rhonchi. Serum C-reactive protein was 75 mg/L and procalcitonin (PCT) was 22 ng/mL. CT in the chest revealed acute lung inflammation (Figure 3); CSF was abnormal, and anti-NMDAR antibodies were positive (titer: 1/80) (Table 1). Herpes simplex virus (HSV) DNA polymerase chain reaction (PCR) in the CSF was negative. Acinetobacter baumanii was found in the sputum culture. A relapse of anti-NMDAR encephalitis combined with pneumonia caused by acinetobacter baumannii was diagnosed, and a cycle of steroid and IVIG pulse therapy were administered once again with antibacterial agents as well as other supportive therapies. There was no positive detection in tumor screening. The patient gradually recovered except for mild psychiatric sequelae.
In our case, ANMDARE relapsed and the condition of the patient got worse following acinetobacter baumannii pneumonia, similar to that with possible mycoplasma pneumoniae infection in a previous study[2]. In recent reports[4,6], pneumonia was seen from 55% to 65% of patients with ANMDARE, more likely to accompany with status epilepticus, and was regarded as one of the risk factors influencing the outcome of this disease. The Chinese cohort study have firstly focused on and explore the causes of death of patients with ANMDARE, revealing that all the patients died with the existence of pneumonia[6]. Complications such as severe pneumonia, multiple organ dysfunction syndrome (MODS), refractory status epilepticus (RSE) and sepsis were the main causes of death of patients with ANMDARE[1,4,6]. Acinetobacter baumannii pneumonia may be a direct infectious complication of the use of immunosuppressant and may result in a fatal episode of respiratory failure or sepsis. But this has rarely been described in patients with ANMDARE. It is fairly clear that the occurrence of acinetobacter baumannii pneumonia has provoked the relapse and aggravation of ANMDARE in our case. However, the mechanism of the underlying association is not clear, and whether this connection is meaningful warrants further study. There is increasing evidence that ANMDARE might develop in the context of viral encephalitis, and in term of etiology, a viral trigger (e.g., herpes simplex) might be considered in the synaptic autoimmunity[5,7]. Moreover, the frequency of ANMDARE is likely to be four times higher than HSV encephalitis in young individuals in the California Encephalitis Project[8]. Approximately 4.4% of encephalitis with unclear cause are anti-NMDAR encephalitis, followed by HSV type 1, enterovirus, varicella zoster virus, and West Nile virus[8]. The correlation between ANMDARE and viral etiology is important[5]. Whether the lung infection could be a trigger of ANMDARE and its mechanism need further studies.
Although the clinical spectrum of ANMDARE was widely reported, the disease often cannot be diagnosed earlier till the later clinical courses due to the nonspecific nature of symptoms and insufficient awareness of the disorder[9] in most departments except the neurology, especially in the emergency department. There are several challenges in managing ANMDARE: The outcome is poor among developing countries, including China, due to the knowledge gap compared with developed countries, delays in diagnosis and misdiagnosis, complications as well as economic affordability. While approximately 80% of patients have good outcome[3,10], ANMDARE carries a significant morbidity and mortality because of life-threatening illnesses such as intractable seizure, ventilator care, rhabdomyolysis or pneumonia[4,10,11]. Patients could die of MODS during hospitalization, even after immunotherapy[12]. Moreover, a minority of patients received second-line immunotherapy. It is important to make decisions regarding instance to facilitate a second-line immunotherapy and therapy timing typically based on symptoms, doctors’ experience, therapeutic response, or patients’ preference. Furthermore, many patients were discharged before they fully recovered due to the financial burden or ward beds shortage in hospital. While being back home or transferred to a primary care center, the condition may worse due to the progress of the disorder or complications including lung infection, urinary infection, rhabdomyolysis, venous thrombosis and hypersexuality[4,10,13]. In China, awareness and knowledge of this disorder among not only neurologists but also psychiatrists and primary care physicians, should be extended and taken into consideration in the differential diagnosis when they encounter patients with a new onset of neurologic complaints preceded by psychiatric symptoms. Meanwhile, the popularity and feasibility of test method of NMDAR-Abs are required in most hospitals in China, so that physicians can more readily identify those affected with this potentially treatable disorder, facilitating earlier treatment and good outcome.
In conclusion, acinetobacter baumannii pneumonia can aggravate the process of ANMDARE. The mechanism underlying the association remains unknown and needs further studies. In China, awareness and knowledge of this disorder associated with lung infection should be extended, especially in the emergency medicine community.
A 27-year-old male with status epilepticus.
Anti-N-methyl-D-aspartate receptor encephalitis (ANMDARE).
Viral encephalitis.
Elevated titers of antibody against NMDAR in the cerebrospinal fluid.
Magnetic resonance imaging of the brain revealed no abnormal changes.
Steroid and intravenous immunoglobulin pulse therapy.
About 30 cases of NMDAR encephalitis after acinetobacter baumannii pneumonia.
ANMDARE developed multiple psychiatric and neurological features in the clinical course, including psychosis, epileptic seizures, autonomic instability, dyskinesia, decreased consciousness, and hypoventilation. It predominantly affects young adults and children with or without tumors and well responds to immunotherapy.
This case report shows ANMDARE that aggravates after acinetobacter baumannii pneumonia. It’s rarely reported.
This is an interesting case report.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Peluso I, Tan XR S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
| 1. | Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2002] [Cited by in RCA: 1726] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 2. | Gable MS, Gavali S, Radner A, Tilley DH, Lee B, Dyner L, Collins A, Dengel A, Dalmau J, Glaser CA. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis. 2009;28:1421-1429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2136] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 4. | Huang Q, Wu Y, Qin R, Wei X, Ma M. Clinical characteristics and outcomes between children and adults with anti-N-Methyl-D-Aspartate receptor encephalitis. J Neurol. 2016;263:2446-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Gilbert GJ. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. 2014;82:2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Chi X, Wang W, Huang C, Wu M, Zhang L, Li J, Zhou D. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand. 2017;136:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Ellul MA, Griffiths MJ, Iyer A, Avula S, Defres S, Baborie A, Vincent A, Martin NG, Sadarangani M, Pollard AJ. Anti-N-Methyl-D-Aspartate Receptor Encephalitis In A Young Child With Histological Evidence On Brain Biopsy Of Coexistent Herpes Simplex Virus Type 1 Infection. Pediatr Infect Dis J. 2016;35:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 516] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 9. | Barry H, Byrne S, Barrett E, Murphy KC, Cotter DR. Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull. 2015;39:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Wang W, Li JM, Hu FY, Wang R, Hong Z, He L, Zhou D. Anti-NMDA receptor encephalitis: clinical characteristics, predictors of outcome and the knowledge gap in southwest China. Eur J Neurol. 2016;23:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Lim JA, Lee ST, Kim TJ, Moon J, Sunwoo JS, Byun JI, Jung KH, Jung KY, Chu K, Lee SK. Frequent rhabdomyolysis in anti-NMDA receptor encephalitis. J Neuroimmunol. 2016;298:178-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Liu J, Wang D, Xiong Y, Liu B, Liu M. Anti-NMDAR Encephalitis of 11 Cases in China - Detailed Clinical, Laboratory and Imagiological Description. Eur Neurol. 2015;74:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Sakamoto H, Hirano M, Samukawa M, Ueno S, Maekura S, Fujimura H, Kuwahara M, Hamada Y, Isono C, Tanaka K. Details of treatment-related difficulties in men with anti-N-methyl-D-aspartate receptor encephalitis. Eur Neurol. 2013;69:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |