Published online Aug 16, 2017. doi: 10.12998/wjcc.v5.i8.324
Peer-review started: March 29, 2017
First decision: May 8, 2017
Revised: May 16, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: August 16, 2017
Processing time: 140 Days and 13.2 Hours
Sepsis is one of the major challenges of today. Although gram-positive bacteria related infections are more prevalent in hospital setting, the highest mortality rate is associated with gram-negative microorganisms especially Enterobacteriaceae. Enterobacteriaceae, including Escherichia coli, Klebsiella spp., Proteus spp., Enterobacter spp. and Serratia spp. Resistance to β-lactams in Enterobacteriaceae is primarily attributed to the production of B-lactamase enzymes with subsequent antibiotic hydrolysis and to a lesser extent by alteration of efflux pump or porins expression. Carbapenem resistant Enterobacteriaceae (CRE) and Acinetobacter baumannii are the most notorious pathogens due to the high incidence of morbidity and mortality especially in the immunocompromised patients in the intensive care unit. The most appropriate antimicrobial therapy to treat CRE is still controversial. Combination therapy is preferred over monotherapy due to its broad-spectrum coverage of micro-organisms, due to its synergetic effect and to prevent development of further resistance. Current suggested therapies for CRE resistance as well as promising antibiotics that are currently under investigation for winning the war against the emerging CRE resistance are reviewed and discussed.
Core tip: Carbapenem resistant Enterobacteriaceae (CRE) is the most notorious pathogens contributing to a significant morbidity and mortality rate in septic patients especially in the intensive care unit. The most appropriate antimicrobial therapy to treat CRE is still controversial. This review is conducted to discuss the effectiveness of available therapies at this moment and to elaborate on different promising drugs that are still under investigation in order to win the combat on rising antimicrobial resistance.
- Citation: Alhashem F, Tiren-Verbeet NL, Alp E, Doganay M. Treatment of sepsis: What is the antibiotic choice in bacteremia due to carbapenem resistant Enterobacteriaceae? World J Clin Cases 2017; 5(8): 324-332
- URL: https://www.wjgnet.com/2307-8960/full/v5/i8/324.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i8.324
Sepsis is a global healthcare problem and one of the major challenges that health care practitioners face worldwide[1]. It has become one of the major causes of death and its incidence is continuing to rise making it a huge burden in terms of increased morbidity and mortality, prolonged hospital stay, increased risk of having antimicrobial resistance and increasing hospital cost. It was estimated that the incidence of septic cases increased 13.7% each year over a period of 22 years[2-4]. Sepsis is newly re-defined as a life-threatening organ dysfunction due to a dysregulated host response to infection. Septic shock is defined as a subset of sepsis in which particularly profound circulatory, cellular and metabolic abnormalities substantially increase mortality[5].
Sepsis is a medical emergency; hence, antimicrobial treatment should be started as soon as sepsis is suspected. To prevent development of further complications and progression of the patient into septic shock and multi organ failure, profound knowledge of the causative pathogens is needed to select the proper antibiotic treatment. To reduce sepsis associated mortality, it has been widely advocated to start empirical antibiotic therapy from the first hour following sepsis identification. This strategy leads to a reduction in mortality of 13.7%[6-8]. The most common primary site involved in sepsis is the respiratory tract, mainly pneumonia, followed by genitourinary tract infections. Other sites involved are the abdomen, wound and soft tissue infections, the central nervous system (CNS) and the cardiovascular system. In some cases, the source origin is unknown (Table 1)[6,9,10]. However, the most commonly isolated pathogens depend on the infection site. In wound infections Staphylococcus aureus and coagulase negative staphylococci was found to be the most causative organism of both meningitis and pneumonia, while Escherichia coli is the most prevalent cause of urinary tract infections (UTI) related sepsis[11,12]. Regarding blood stream infection (BSI), coagulase negative staphylococci and E. coli are the notable organisms isolated[10].
| Site of infection | Mortality (%) |
| Blood stream infection | 34.2 |
| Respiratory | 22 |
| Genitourinary | 8.2 |
| Wound and soft tissue infection | 10.55 |
| Abdomen | 10.25 |
| CNS | 17.4 |
| Device related | 9.5 |
| Endocarditis | 25.95 |
| Others | 7.05 |
In the intensive care unit (ICU) setting, the most common isolated pathogens causing severe sepsis are S. aureus followed by Pseudomonas infection, Enterobacteriaceae and fungal infection, respectively. Acinetobacter baumannii was involved in 9% of all infections[10]. But in general, gram negative pathogens are the most commonly found in sepsis patients[2,4,11,13].
Although gram-positive bacteria related infections (especially Staphylococcus) are more prevalent in the hospital setting (62.2%), the highest mortality rate is associated with gram-negative micro-organisms. Enterobacteriaceae are the most common microorganisms in gram-negative sepsis[14].
Enterobacteriaceae, a family of Gram negative pathogens, includes E. coli, Klebsiella spp., Proteus spp., Enterobacter spp. and Serratia spp. These organisms account for half of the bacteremia’s that are usually caused by overspill of bacteria from their primary sites[14]. E. coli and K. pneumoniae, in particular, are the major community and hospital acquired pathogens that usually cause intra-abdominal infections, urinary tract infections and primary bacteremia[15]. The emergence of multi-drug resistant microorganisms has become one of the most important hazards that health care is facing worldwide. Development of such resistance can be attributed to many risk factors including previous ICU admission, presence of a central venous catheter especially in hemato-oncology patients who are receiving chemotherapy, presence of an indwelling catheter insertion, prolonged use of antibiotics, prolonged hospital stay, hospitalization in an area endemic for multidrug resistance (MDR) and a history of previous colonization or infection with these microorganisms[16,17]. Resistance to β-lactams in Enterobacteriaceae is primarily attributed to the production of B-lactamase enzymes with subsequent antibiotic hydrolysis and to a lesser extent by alteration of efflux pump or porins expression[18]. One of the main causes of Enterobacteriaceae resistance is extended spectrum beta-lactamases (ESBL) production. ESBLs are plasmid encoded enzymes that are able to hydrolyze penicillins, broad-spectrum cephalosporins with an oxyiminoside chain, e.g., cefotaxime, ceftazidime and ceftriaxone as well as oxyimino-monobactams such as azetreonam. However, they are ineffective against cephamycins or carbapenems and their antimicrobial activity is inhibited by clavulanic acid[19,20]. The main problem concerning ESBL-producing bacteria is that they usually acquire multiple antimicrobial resistance mechanisms causing not only resistance to cephalosporins but also to aminoglycosides and fluoroquinolones, which further narrows the choices of finding an effective therapeutic agent[21].
Regrettably, these organisms belonging to the β-lactamases producing Enterobacteriaceae have acquired new genetic mutations and became resistant to carbapenem antimicrobial therapy.
These carbapenemase producing Enterobacteriaceae are classified into group A, B and D. β-lactamases micro-organisms based on the type of gene mutation (Table 2). Class A and D have a serine based hydrolytic mechanism, but class B consists of metallo- β-lactamase[22-24].
| Class | Genetic mutation | Clavulanic acid inhibition |
| Class A | Chromosomal encoded (NmcA, Sme, IMI-1, SFC-1) | Partially inhibited |
| Plasmid encoded (IMI-2, GES, KPC) | ||
| Class B | Metallo-β-lactamase (IMP, VIM and NDM-1, SIM, GIM, SPM) | Resistant to clavulanic acid |
| Class D | Plasmid encoded oxa-48 | Resistant to clavulanic aci |
Carbapenem resistant Enterobacteriaceae (CRE) and A. baumannii are the most notorious pathogens due to the high incidence of morbidity and mortality especially in the immunocompromised patients in the ICU. The incidence of CRE was reported in various countries worldwide including East Asia, India, USA and many European countries. A multicenter study, conducted in Shanghai, China, revealed a high proportion of ESBL type E. coli as a cause of bloodstream infections. In addition, it was noted that the most common involved gene was CTX-M (CTX-M-15 CTX-M-14 and CTX-M-55, respectively). No carbapenemases producing Enterobacteriaceae were reported[25]. On the contrary, in Turkey a retrospective study evaluating ICU patients over ten years, showed that antibiotic resistance most dramatically increased due to carbapenem resistant A. baumannii followed by Pseudomonas spp, E. coli and K. pneumoniae, respectively. In addition, a reduction in methicillin resistant S. aureus (MRSA) prevalence from 96% to 54% was noticed[26]. In the United States, it was reported in CRE outbreaks that resistance, especially to K. pneumoniae and to a lesser extent other Enterobacteriaceae, were important in CRE resistance[27].
A case control study in New York showed that the majority of deaths due to bacteremia in neutropenic oncology patients were caused by CRE infections in 53%, of which gram negative Enterobacteriaceae was found in 13%-18% of the patients. Independent risk factors for increased CRE susceptibility were: Previous use of β -lactam antibiotics (e.g., 3rd or 4th generation cephalosporins or carbapenems) within the last 30 d; receiving trimethoprim-sulfamethoxazole or glucocorticoids at the time of onset of blood stream infection and having previous CRE infection isolate[27]. In order to avoid development of more resistance, it is crucial to be selective in the choice of antibiotics. Choosing the proper antibiotic regimen should be based on clinical findings supported by rapid diagnosis[22].
Goodman et al[16] tried to develop a clinical decision tree to predict whether a patient with bacteremia was infected with an ESBL-producing pathogen. They retrospectively studied a cohort of patients with bacteremia in Johns Hopkins hospital to identify clinical criteria to diagnose those patients with ESBL bacteremia, especially gram negative Enterobacteriaceae, to avoid misuse of antibiotics in the future. The clinical criteria were: A history of ESBL colonization or infection in the last 6 mo with chronic usage of indwelling venous catheter or dialysis, patient age ≥ 43 years, recent hospitalization in an ESBL high-burden area and a history antibiotic use for ≥ 6 d in the previous 6 mo. They made a clinical decision based on these five yes-or-no questions. When the patient had a history of ESBL colonization or infection in the last 6 mo with chronic usage of indwelling venous catheter or dialysis they had a 92% chance of being ESBL positive. With these criteria, positive predictive value was 90.8% and negative predictive value was 91.9%. In addition, they found that 43% of patients with an ESBL positive culture had received chemotherapy in the recent history.
Another score has been developed to identify patients with high suspicion of CRE or ESBL-BSI so antibiotic therapy might be started on time to reduce mortality (Table 3). Risk factors were chemotherapy in the last 3 mo, foreign invasive device, absence of peripheral vascular disease, reduced level of consciousness, hospitalization of > 3 d and age < 65 years old. With a total score ≥ 32 patients were considered as high risk for CRE BSI infections and required antimicrobial therapy targeted for CRE BSI infections. Although this test showed a lower sensitivity and specificity, its negative predictive value might prevent needless use of toxic antibiotics[28].
| Risk factor | Score (points) |
| History of chemotherapy in the last 3 mo | 19 |
| Invasive devices | 10 |
| Absence of peripheral vascular disease | 10 |
| Impairment of level of consciousness at the time of illness | 9 |
| Hospitalization for 3 or more days before development of BSI | 7 |
| Age < 65 years old | 6 |
The Carba NP test is such a rapid diagnostic with a very high sensitivity and specificity that can differentiate between class A, B and C CRE. In addition, other promising tests such as PCR assay and matrix assisted laser desorption ionization might facilitate CRE diagnosis. In addition, fast gram-negative blood culture assays and film array blood culture can help in identifying carbapenemase genes within two hours[29,30]. However, deciding on the most appropriate antibiotic choice in bacteremia due to CRE can be very challenging.
The most appropriate antimicrobial therapy to treat CRE is still controversial. No consensus has been reached regarding the optimal choice of antibiotic therapy. As a consequence of the evolving broad spectrum antibiotic resistance, the old fashioned antibiotics, previously discarded because of their side effects, e.g., polymyxin E (colistin), polymyxin B, aminoglycosides and fosfomycin, have reappeared, because of effectiveness to some extent[31,32]. Despite the numerous publications on the subject, no consensus has been reached even on the preference of monotherapy or combination therapy[33].
In a study conducted in a mice sepsis model, no significant difference was shown between colistin monotherapy and tigecycline monotherapy in treating carbapenem-resistant K. pneumoniae. In addition, combination of both colistin and tigecycline did not show superiority over monotherapy[34]. However, several studies suggested that combination therapy might be superior to monotherapy in terms of mortality rates. A review conducted by Falagas et al[35] described a cohort of 692 patients in which majority had confirmed Klebsiella pneumoniae carbapenemase producing K. Pneumoniae (KPC-KP) isolates and most of them related to bacteraemia. Mortality rate among those who received combination antimicrobial therapy ranged from 50% to 67% with the lowest rate associated with combination of tigecycline and gentamicin and highest rate with colistin and carbapenem (50% mortality rate in combination of tigecylcine with gentamicin, 64% in tigecycline-colistin and 67% for carbapenem-colistin combination). Patients who received colistin monotherapy had a mortality rate of 57% and patients who received tigecycline monotherapy 80%. The superiority of using combination therapy was shown in only three studies. No hard conclusions can be drawn from this review to decide on the use of single agent or combined therapy. Except in the critically ill patients, it is preferable to use combination therapy with superiority of tigecycline and gentamicin as a first choice, but randomized clinical trials need to confirm this statement.
But even with combination therapy, mortality rate remains high in patients with CRE related bacteremia. The underlying illness of the patient remains the most important risk factor for mortality. Tumbarello et al[36] showed the lowest mortality rate in patients with KPC-KP related sepsis using combined antimicrobial regimens especially those containing meropenem (when MIC ≤ 8 mg/L).
In a retrospective cohort study by Tumbarello et al[36] it was shown that combined therapy was superior to monotherapy in treating KPC-KP infection. Survival in patients with blood stream infection (mainly septic patients) with KPC-KP receiving monotherapy had a mortality rate of 54.3% while those receiving a combination therapy had a mortality rate of 34.1%. The same study demonstrated that a combination of tigecycline or colistin with meropenem had a better outcome compared to other combination therapies (12.5% mortality rate compared to 16.6% for tigecyclin, gentamicin with meropenem, 57% for colistin-gentamicin, 50% for tigecyclin-gentamicin and 30.4% tigecycline and colistin combination therapy).
Interestingly, in a prospective study by de Maio Carrilho et al[37] in patients with infections caused by KPC-KP (but also Enterobacteriaceae and E. coli) regimens of three or more antibiotics did not show any improved survival in comparison to regimens with two antibiotics. Moreover, monotherapy was just as effective as combination therapy in patients with UTI. Other independent risk factors such as dialysis, older age and septic shock seem to influence patient outcome more than monotherapy vs combination therapy.
Nevertheless, taken all current evidence into account, combination therapy can have a significant association with a lower mortality rate and increase the cure rate compared to monotherapy in the septic patient since each drug has its own mechanism of action, which can create a synergistic environment while combating resistant bacterial strains. The limited number of antimicrobial agents currently available to treat CRE will be further discussed in this review.
Colistin is one of polymyxin antibiotics with bactericidal activity against Gram-negative bacterial infection[38]. Although the usage of this antibiotic was banned for many years due to its nephrotoxicity and neurotoxicity effect, it was reintroduced again due to emergence of MDR microorganisms. However, the use of colistin in treating CRE infection is still controversial[39].
Qureshi et al[40] retrospectively evaluated a cohort of 41 patients admitted to the ICU from two different hospitals in the United States with almost similar clinical and demographic variables. Of the 41 patients who developed bacteremia with a KPC-producing K. pneumoniae, seven patients died before initiating treatment. Among those who received combination therapy with carbapenem/tigecycline or carbapenem/colistin 28 d survival was significantly higher than in those on monotherapy (2 out of 15 patients receiving combination therapy died compared to 11 out of 19 patients receiving monotherapy).
Regarding the optimal dose for colistin, Gibson et al[41] showed that use of high dose colistin (> 4.4 mg/kg per day) in patients with CRE bacteremia was associated with a better clinical outcome, i.e., reduction of leucocyte < 12000 cells/mm3, no fever for 48 h and hemodynamically stable without any vasopressor. Also a better microbiological outcome was demonstrated, i.e., eradication of CRE on day 7 after starting colistin.
Unfortunately, although still rare, colistin resistant CRE species are emerging in China, United States and different European countries. It is most often observed in Enterobacteriaceae harboring the mcr-1 gene along with carbapenemase resistant gene[42-44].
Despite the fact that some investigators showed that development of colistin resistant CRE did not correlate with an increased mortality compared to patients without colistin resistant CRE[37], others have found that having colistin resistant KP is an independent risk factor of death especially in those with bacteremia[36,43].
Colistin seems to be a good alternative in vulnerable patients (without any evidence of renal impairment) especially when combined with other antibiotics such as carbapenems. However, it is very important to pay extra-attention for colistin resistant strains.
Despite the fact of developing resistance to carbapenems anti-microbial therapy, they still can be of use especially in combination with other antimicrobial agents in colistin resistant Enterobacteriaceae. In a review by Bassetti et al[45], it was recommended that patients with KPC-KP and a MIC of isolate between 8 mg/L-16 mg/L or < 8 mg/L should receive a high dose of carbapenem containing therapy with a prolonged infusion together with colistin, tigecycline or an aminoglycoside. The underlying reason was prevention of developing new resistance to the rest of CRE antimicrobial therapy
In a case report on ertapenem and meropenem combination therapy, it was reported that an elderly patient with KPC-producing E. coli isolated from surgical site and nosocomial pneumonia with a contra-indication to colistin use due to a recent renal transplantation was started on combination therapy with ertapenem and meropenem showing good response. Unfortunately, the patient died later due to hemorrhagic shock[46].
In order to have a better outcome in colistin resistant Enterobacteriaceae, double carbapenem treatment was introduced. In two patients with carbapenemase-producing K. pneumoniae who were also colistin resistant, a combination of meropenem 2 g every 8 h and ertapenem 1 g every 24 h were given. In a third patient, dosages were adjusted for renal function. Both patients showed clinical improvement and also in vitro bactericidal activity was maintained up to 24 h. In conclusion, in such select cases like resistance to colistin, where options to treat CRE related sepsis are limited, a combination of two carbapenem antibiotics could be beneficial[47].
In a trial performed by Cprek et al[48] ertapenem/carbapenem double therapy (consisting of one gift of 1 g ertapenem given daily 1 h before administration of meropenem 2 g or doripenem 500 mg and the rest of the daily doses of meropenem or doripenem given normally) a favorable outcome was observed with maximum benefit in patients with CRKP bacteremia (43%) followed by pneumonia, intraabdominal, UTI and skin associated CRE infections.
Taking these observations into account, a combination of two carbapenems can be effective and even superior to other combination regimens in bacteremia patients due to its synergistic effect.
Tetracyclines are a group of antibiotics that exhibit bacteriostatic activity by reversible binding to 30S ribosomes that interfere with protein synthesis. It is widely used due to its coverage of both gram-positive and gram-negative bacteria as well as some anaerobes and parasites[49]. Tigecycline is a glycylcycline, which is a tetracycline derivative and exhibits broad spectrum activity covering many organisms including MDR pathogens[50]. Many studies were conducted to evaluate the efficacy of tetracyclines mainly tigecycline in treating CRE related sepsis. A systematic review demonstrated that tigecycline did not significantly improve sepsis related mortality[51]. However, combination therapy containing tigecycline did significantly improve survival. In addition, it was found that administration of high dose regimen was associated with better outcome compared to standard dose tigecycline in combination therapy.
Administration of tigecycline monotherapy was associated with a high mortality rate[52]. Tigecycline might have a role in the treatment of CRE if it is used as a part of combination therapy particularly with aminoglycoside group or colistin[52]. Other tetracyclines, minocycline and eravacycline, are also studied in the treatment of CRE. Although high dose intravenous minocycline (200 mg twice daily) can be effective in carbapenem resistant A. baumannii up to 74%, its efficacy is less when dealing with CRE with only 12% of the CRE susceptible to minocycline[53]. Another promising agent is eravacycline, belonging to the fluorocyclines. In vitro it showed a potent effectiveness to MDR organisms[54]. An ongoing phase 3 clinical trial with eravacycline (GNITE4) might give us more data on its effectiveness[55]. The preliminary results suggest that there is no place for the use of tetracyclines in the treatment of CRE related sepsis; however the definitive results have not been published until now.
The antibiotic group of aminoglycosides consists of gentamicin, amikacin, streptomycin, paromomycin, streptomycin and plazomicin, the last one still under clinical research. This group covers mainly gram negative and to some extent gram-positive pathogens and Mycobacteria[56]. It is usually used in combination with other antimicrobial agents in serious infections due to its synergistic effect[57]. The major side effects are ototoxicity and nephrotoxicity together with its narrow therapeutic window it can limit its use[58]. Aminoglycosides (especially gentamicin and amikacin) have shown their efficacy in treating carbapenem resistant KP UTI compared to other carbapenem resistant antimicrobial treatment (88% clearance compared to tigecycline and colistin), but its role in carbapenem resistant bacteremia as monotherapy is still uncertain[58]. A retrospective cohort study demonstrated that administration of gentamicin is an independent factor, which can improve 30 d mortality mainly in cases of KPC-KP related sepsis.
Especially in patients with both Carbapenem and colistin resistant KP, it decreased the mortality rate to 20.7% in comparison to 61.9% in patients treated with non-gentamicin containing therapy[59].
In conclusion, the role of aminoglycosides, mainly gentamicin, was studied previously particularly in combination with tigecycline with promising results. Now, it can be considered as a good option in the CRE patient with sepsis. Regarding the rest of aminoglycoside group antibiotics, further prospective studies are recommended.
The same might be applied for fosfomycin which has shown only its effectiveness in the treatment of patients with CRE UTI, but up until now no sufficient data exists supporting the use of fosfomycin either as a monotherapy or as part of a combination therapy in treating sepsis[58]. In a study by Bowers et al[60], 68 patients were treated with intravenous fosfomycin either in combination with colistin or with tigecycline. Effectiveness was demonstrated in 54.2% of patients at day 14. Mortality rate was 37.5% at day 28, with the highest mortality in having bacteremia; ventilator associated pneumonia and CRE KP isolates or P. aeruginosa isolates. Interestingly, three patients developed fosfomycin resistance shortly after treatment.
Alternative combination regimens can be considered in the future as curative agents in CRE related sepsis, but further clinical trials and prospective studies are required to assess its effectiveness. Fortunately, many new promising drugs might win the current battle against the current CRE resistance. One of them is plazomicin that belongs to the group of aminoglycosides. Recently, a phase 3 clinical trial was published showing efficacy of plazomicin. It was demonstrated that plazomicin significantly reduced mortality rate and reduced complications in patients with severe infection including CRE bacteremia, ventilation associated pneumonia and hospital acquired pneumonia related to CRE compared to colistin (28 d mortality rate was 11.4% in the plazmicin group compared to 40% in the colistin group). It also showed that plazomicin has the same efficacy of ertapenem in treating UTI[61], therefore it might play an important role in ceftazidime-avibactam combination therapy. In a recent study by Wu et al[62], three patients with CRE bacteremia (one patient with septic shock and one patient with suspected endocarditis) were successfully treated with ceftazidime-avibactam combination therapy. Combination of ceftazidime-avibactam is effective against oxa-48 and KP CRE[63].
Another option in the battle of CRE might be the addition of vabobactam to meropenem. Vabobactam is a boronic acid beta-lactamase inhibitor, which acts mainly on the serine carbapenamase and it has shown its efficacy in treating complicated UTI including those caused by CRE in phase 1 and 2 trials. A phase 3 trial is still ongoing now. In addition, its role in treating bacteremia needs further investigation[64].
In addition, combination of relebactam with imipenem and cilastatin is a promising option. Relebactam belongs to same group of vabobactam antibiotics. A phase 3 trial is designed to compare this combination with piperacillin/tazobactam in the treatment of complicated UTI. Its role in treatment of bacteraemia has not been investigated up until now[65].
One of the promising discoveries that can help in reducing the rate of CRE resistance is the peptide-conjugated phosphorodiamidate morpholino oligomer. It is a neutral DNA analogue which can inhibit gene expression of carbapenemases. It has been demonstrated that PRMO can target NDM1 (class B carbapenemases). It was shown that in combination with meropenem, it improved patient survival up to 92% because it re-established meropenem function[66].
The most appropriate antibiotics to treat CRE related sepsis is still debatable. Combination therapy is preferred over monotherapy in most of the studies due to its broad-spectrum coverage of organisms, its synergetic effect and to prevent development of further resistance.
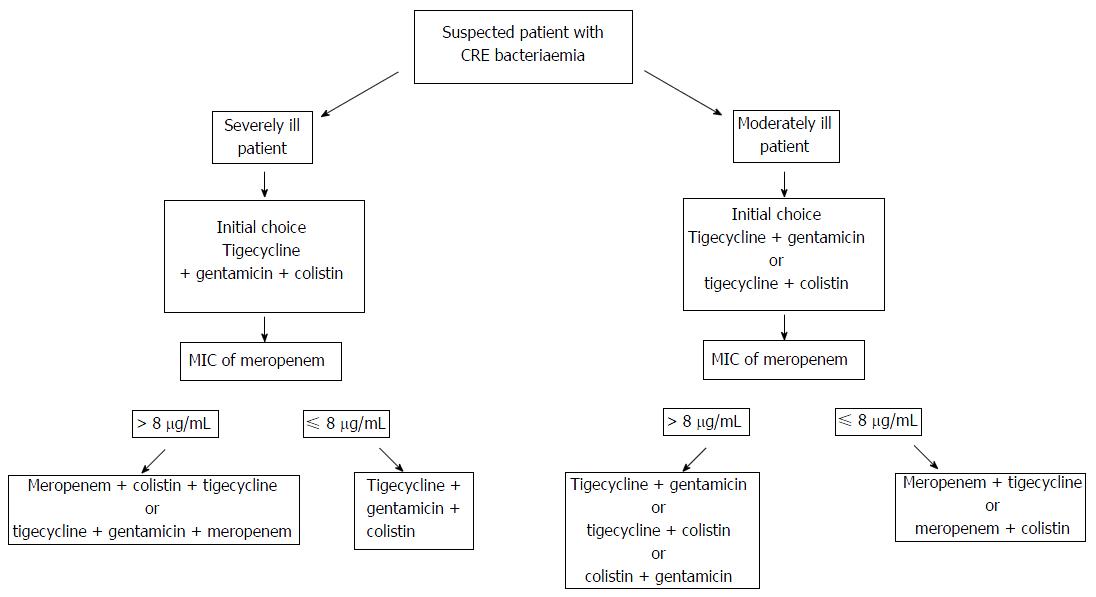
In severely ill patients with co morbidities, a combination of two or more antibiotics is preferred. One of the best treatments up until now has been a combination of meropenem, tigecycline and colistin. A second option might be the combination therapy with tigecycline, gentamicin and meropenem. In moderately ill patients, it is recommended to administer the combination of tigecycline and gentamicin. If the MIC is less than 8 μg/mL, it is advisable to switch to a carbapenem containing therapy. In case of colistin resistance, a combination of two carbapenems can be used (e.g., ertapenem with meropenem or ertapenem with doripenem) besides the combinations shown in the algorithm (Figure 1). Many promising antibiotics are currently under investigation. The most optimal treatment still needs to be determined to win the battle against the emerging CRE resistance.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Akbulut S, Desai DJ, Mehdi I, Vij M S- Editor: Ji FF L- Editor: A E- Editor: Wang S
| 1. | Stehr SN, Reinhart K. Sepsis as a global health problem-why we need a global sepsis alliance. Shock. 2013;39 Suppl 1:3-4. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546-1554. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 4208] [Cited by in RCA: 4254] [Article Influence: 193.4] [Reference Citation Analysis (0)] |
| 3. | Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011;62:1-8. [PubMed] [Cited in This Article: ] |
| 4. | Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care. 2014;18:596. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 15803] [Cited by in RCA: 16252] [Article Influence: 1805.8] [Reference Citation Analysis (2)] |
| 6. | Ferrer R, Martin-Loeches I, Phillips G, Osborn TM, Townsend S, Dellinger RP, Artigas A, Schorr C, Levy MM. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014;42:1749-1755. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in RCA: 1008] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 7. | Daniels R. Surviving the first hours in sepsis: getting the basics right (an intensivist’s perspective). J Antimicrob Chemother. 2011;66 Suppl 2:ii11-ii23. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38:1045-1053. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 652] [Cited by in RCA: 664] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 9. | Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4-11. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 699] [Cited by in RCA: 835] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 10. | Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303:2495-2503. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Ayub M. Isolation of Pathogens Causing Sepsis, Pus and Infected Wounds from Critical Care Unit: A Retrospective Study. Ann Clin Lab Res. 2015;3:4. [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Chamberlain NR. Sepsis and Septic Shock. Available from: https://www.atsu.edu/faculty/chamberlain/website/lectures/lecture/sepsis2007.htm. [Cited in This Article: ] |
| 13. | Chong D. Sepsis and Septic Shock. September 21. 2007; Available from: http://www.columbia.edu/itc/hs/medical/pathophys/id/2009/sepsisNotes.pdf. [Cited in This Article: ] |
| 14. | Wilson J, Elgohari S, Livermore DM, Cookson B, Johnson A, Lamagni T, Chronias A, Sheridan E. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect. 2011;17:451-458. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Donnenberg MS. Enterobacteriaceae. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. United States: Elsevier 2010; 2815-2834. [Cited in This Article: ] |
| 16. | Goodman KE, Lessler J, Cosgrove SE, Harris AD, Lautenbach E, Han JH, Milstone AM, Massey CJ, Tamma PD; Antibacterial Resistance Leadership Group. A Clinical Decision Tree to Predict Whether a Bacteremic Patient Is Infected With an Extended-Spectrum β-Lactamase-Producing Organism. Clin Infect Dis. 2016;63:896-903. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Paño-Pardo JR, López Quintana B, Lázaro Perona F, Ruiz Carrascoso G, Romero-Gómez MP, Loeches Yagüe B, Díaz-Pollán B, Martínez-Virto A, Mingorance J, García Rodríguez J. Community-Onset Bloodstream and Other Infections, Caused by Carbapenemase-Producing Enterobacteriaceae: Epidemiological, Microbiological, and Clinical Features. Open Forum Infect Dis. 2016;3:ofw136. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Gazin M, Paasch F, Goossens H, Malhotra-Kumar S; MOSAR WP2 and SATURN WP1 Study Teams. Current trends in culture-based and molecular detection of extended-spectrum-β-lactamase-harboring and carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2012;50:1140-1146. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18:657-686. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 2039] [Cited by in RCA: 2204] [Article Influence: 110.2] [Reference Citation Analysis (0)] |
| 20. | Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933-951, table of contents. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1579] [Cited by in RCA: 1611] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 21. | Díaz PQ, Bello HT, Domínguez MY, Trabal NF, Mella SM, Zemelman RZ, González GR. [Resistance to gentamicin, amikacin and ciprofloxacin among nosocomial isolates of klebsiella pneumoniae subspecie pneumoniae producing extended spectrum beta-lactamases]. Rev Med Chil. 2004;132:1173-1178. [PubMed] [Cited in This Article: ] |
| 22. | Nordmann P, Naas T, Poirel L. Global Spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791-1798. [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 1551] [Cited by in RCA: 1677] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 23. | Davies TA, Marie Queenan A, Morrow BJ, Shang W, Amsler K, He W, Lynch AS, Pillar C, Flamm RK. Longitudinal survey of carbapenem resistance and resistance mechanisms in Enterobacteriaceae and non-fermenters from the USA in 2007-09. J Antimicrob Chemother. 2011;66:2298-2307. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67:1597-1606. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in RCA: 680] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 25. | Wang S, Zhao SY, Xiao SZ, Gu FF, Liu QZ, Tang J, Guo XK, Ni YX, Han LZ. Antimicrobial Resistance and Molecular Epidemiology of Escherichia coli Causing Bloodstream Infections in Three Hospitals in Shanghai, China. PLoS One. 2016;11:e0147740. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Alp E, Orhan T, Kürkcü CA, Ersoy S, McLaws ML. The first six years of surveillance in pediatric and neonatal intensive care units in Turkey. Antimicrob Resist Infect Control. 2015;4:34. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Bratu S, Mooty M, Nichani S, Landman D, Gullans C, Pettinato B, Karumudi U, Tolaney P, Quale J. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother. 2005;49:3018-3020. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Leibman V, Martin ET, Tal-Jasper R, Grin L, Hayakawa K, Shefler C, Azouri T, Kaplansky T, Maskit M, Lazarovitch T. Simple bedside score to optimize the time and the decision to initiate appropriate therapy for carbapenem-resistant Enterobacteriaceae. Ann Clin Microbiol Antimicrob. 2015;14:31. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Nordmann P, Poirel L, Dortet L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2012;18:1503-1507. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 619] [Cited by in RCA: 606] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 30. | Dodémont M, De Mendonça R, Nonhoff C, Roisin S, Denis O. Performance of the Verigene Gram-negative blood culture assay for rapid detection of bacteria and resistance determinants. J Clin Microbiol. 2014;52:3085-3087. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Taneja N, Kaur H. Insights into Newer Antimicrobial Agents Against Gram-negative Bacteria. Microbiol Insights. 2016;9:9-19. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Theuretzbacher U, Van Bambeke F, Cantón R, Giske CG, Mouton JW, Nation RL, Paul M, Turnidge JD, Kahlmeter G. Reviving old antibiotics. J Antimicrob Chemother. 2015;70:2177-2181. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment Options for Carbapenem-Resistant Enterobacteriaceae Infections. Open Forum Infect Dis. 2015;2:ofv050. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in RCA: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 34. | Demiraslan H, Dinc G, Ahmed SS, Elmali F, Metan G, Alp E, Doganay M. Carbapenem-resistant Klebsiella pneumoniae sepsis in corticosteroid receipt mice: tigecycline or colistin monotherapy versus tigecycline/colistin combination. J Chemother. 2014;26:276-281. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Falagas ME, Lourida P, Poulikakos P, Rafailidis PI, Tansarli GS. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother. 2014;58:654-663. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 36. | Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, Maiuro G, Tedeschi S, Celani L, Cardellino CS, Spanu T, Marchese A, Ambretti S, Cauda R, Viscoli C, Viale P; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015;70:2133-2143. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 37. | de Maio Carrilho CM, de Oliveira LM, Gaudereto J, Perozin JS, Urbano MR, Camargo CH, Grion CM, Levin AS, Costa SF. A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect Dis. 2016;16:629. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | MacLaren G, Hooper DC, Bloom A. Colistin: An overview. Available from: https://www.uptodate.com/contents/colistin-an-overview. [Cited in This Article: ] |
| 39. | Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333-1341. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1098] [Cited by in RCA: 1217] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 40. | Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108-2113. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 401] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 41. | Gibson GA, Bauer SR, Neuner EA, Bass SN, Lam SW. Influence of Colistin Dose on Global Cure in Patients with Bacteremia Due to Carbapenem-Resistant Gram-Negative Bacilli. Antimicrob Agents Chemother. 2015;60:431-436. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Mediavilla JR, Patrawalla A, Chen L, Chavda KD, Mathema B, Vinnard C, Dever LL, Kreiswirth BN. Colistin- and Carbapenem-Resistant Escherichia coli Harboring mcr-1 and blaNDM-5, Causing a Complicated Urinary Tract Infection in a Patient from the United States. MBio. 2016;7:pii: e01191-16. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in RCA: 173] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 43. | Capone A, Giannella M, Fortini D, Giordano A, Meledandri M, Ballardini M, Venditti M, Bordi E, Capozzi D, Balice MP. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23-E30. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 44. | Yu H, Qu F, Shan B, Huang B, Jia W, Chen C, Li A, Miao M, Zhang X, Bao C. Detection of the mcr-1 Colistin Resistance Gene in Carbapenem-Resistant Enterobacteriaceae from Different Hospitals in China. Antimicrob Agents Chemother. 2016;60:5033-5035. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 45. | Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29:583-594. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 46. | Oliva A, Cipolla A, Gizzi F, D’Abramo A, Favaro M, De Angelis M, Ferretti G, Russo G, Iannetta M, Mastroianni CM. Severe Bloodstream Infection due to KPC-Producer E coli in a Renal Transplant Recipient Treated With the Double-Carbapenem Regimen and Analysis of In Vitro Synergy Testing: A Case Report. Medicine (Baltimore). 2016;95:e2243. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Oliva A, D’Abramo A, D’Agostino C, Iannetta M, Mascellino MT, Gallinelli C, Mastroianni CM, Vullo V. Synergistic activity and effectiveness of a double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae bloodstream infections. J Antimicrob Chemother. 2014;69:1718-1720. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Cprek JB, Gallagher JC. Ertapenem-Containing Double-Carbapenem Therapy for Treatment of Infections Caused by Carbapenem-Resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2015;60:669-673. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Chartwell Pharmaceuticals LLC. Tetracycline Description. Available from: https://www.drugs.com/pro/tetracycline.html. [Cited in This Article: ] |
| 50. | Greer ND. Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent). 2006;19:155-161. [PubMed] [Cited in This Article: ] |
| 51. | Ni W, Han Y, Liu J, Wei C, Zhao J, Cui J, Wang R, Liu Y. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae Infections: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2016;95:e3126. [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943-950. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in RCA: 748] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 53. | Pogue JM, Neelakanta A, Mynatt RP, Sharma S, Lephart P, Kaye KS. Carbapenem-resistance in gram-negative bacilli and intravenous minocycline: an antimicrobial stewardship approach at the Detroit Medical Center. Clin Infect Dis. 2014;59 Suppl 6:S388-S393. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Sutcliffe JA, O’Brien W, Fyfe C, Grossman TH. Antibacterial activity of eravacycline (TP-434), a novel fluorocycline, against hospital and community pathogens. Antimicrob Agents Chemother. 2013;57:5548-5558. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 55. | Dahlman T. Tetraphase Pharmaceuticals Doses First Patient in IGNITE4 Phase 3 Clinical Trial of Eravacycline in cIAI. Tetraphase Pharmaceuticals. Available from: http://ir.tphase.com/releasedetail.cfm?releaseid=993582. [Cited in This Article: ] |
| 56. | Drew RH, Hooper DC, Bloom A. Aminoglycosides. Available from: https://www.uptodate.com/contents/aminoglycosides?. [Cited in This Article: ] |
| 57. | McGrath BJ, Lamp KC, Rybak MJ. Pharmacodynamic effects of extended dosing intervals of imipenem alone and in combination with amikacin against Pseudomonas aeruginosa in an in vitro model. Antimicrob Agents Chemother. 1993;37:1931-1937. [PubMed] [Cited in This Article: ] |
| 58. | van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115-120. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 230] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 59. | Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, Pontes-Moreno A, López-Cerero L, Pascual A, Natera C, Rodríguez M, Salcedo I, Rodríguez-López F. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70:905-913. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 60. | Bowers DR, Huang V. Emerging Issues and Treatment Strategies in Carbapenem-Resistant Enterobacteriaceae (CRE). Curr Infect Dis Rep. 2016;18:48. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | AKAO-G. Achaogen Announces Positive Results in Phase 3 cUTI and CRE Clinical Trials of Plazomicin. 2016; Available from: http://www.nasdaq.com/press-release/achaogen-announces-positive-results-in-phase-3-cuti-and-cre-clinical-trials-of-plazomicin-20161212-00195. [Cited in This Article: ] |
| 62. | Wu G, Abraham T, Lee S. Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Bacteremia. Clin Infect Dis. 2016;63:1147-1148. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Metan G, Akova M. Reducing the impact of carbapenem-resistant Enterobacteriaceae on vulnerable patient groups: what can be done? Curr Opin Infect Dis. 2016;29:555-560. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Laverty B. FDA Grants Fast Track Status for Investigational Antibiotic CARBAVANCE® (meropenem-vaborbactam). Available from: http://www.businesswire.com/news/home/20160 411005581/en/. [Cited in This Article: ] |
| 65. | Merck. Results of Phase 2 Study of Merck’s Investigational Beta-Lactamase Inhibitor Relebactam Presented at ICAAC/ICC 2015 Company Initiates Pivotal Phase 3 Studies Evaluating Relebactam in Combination with Imipenem/Cilastatin for Treatment of Serious Bacterial Infections. Available from: http://www.businesswire.com/news/home/20150918005023/en/Results-Phase-2-Study-Merck’s-Investigational-Beta-Lactamase. [Cited in This Article: ] |
| 66. | Sully EK, Geller BL, Li L, Moody CM, Bailey SM, Moore AL, Wong M, Nordmann P, Daly SM, Sturge CR. Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo. J Antimicrob Chemother. 2017;72:782-790. [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |