Published online Jul 16, 2017. doi: 10.12998/wjcc.v5.i7.286
Peer-review started: February 9, 2017
First decision: April 18, 2017
Revised: May 12, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: July 16, 2017
Processing time: 165 Days and 14.1 Hours
To determine the sensitivity and specificity of high resolution computed tomography (HRCT) in the diagnosis of otosclerosis.
A systematic literature review was undertaken to include Level I-III studies (Oxford Centre for Evidenced based Medicine) that utilised HRCT to detect histology confirmed otosclerosis. Quantitative synthesis was then performed.
Based on available level III literature, HRCT has a relatively low sensitivity of 58% (95%CI: 49.4-66.9), a high specificity, 95% (95%CI: 89.9-98.0) and a positive predictive value of 92% (95%CI: 84.1-95.8). HRCT is better at diagnosing the more prevalent fenestral form of otosclerosis but remains vulnerable to inframillimetre, retrofenestral and dense sclerotic lesions, despite the advent of more advanced CT scanners with improved collimation.
Whilst the diagnosis of otosclerosis remains largely clinical, HRCT remains the gold standard imaging of choice for the middle ear and serves as a useful adjunct to the clinician, helping to delineate extent of disease and exclude other causes.
Core tip: Diagnosis of otosclerosis remains clinical and high resolution computed tomography (HRCT) can be a useful adjunct when assessing the extent of disease and excluding other causes. HRCT of the temporal bones has a high specificity and low sensitivity and is particularly vulnerable to inframillimetre lesions.
- Citation: Kanzara T, Virk JS. Diagnostic performance of high resolution computed tomography in otosclerosis. World J Clin Cases 2017; 5(7): 286-291
- URL: https://www.wjgnet.com/2307-8960/full/v5/i7/286.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i7.286
Otosclerosis is focal osseous dyscrasia of unknown aetiology which predominantly affects only the endochondral bone of the otic capsule in humans[1]. In the Caucasian population the estimated prevalence is between 0.3% and 0.4% but this is thought to be less in the Asian population[2,3]. However, there is a dearth of high level studies evaluating the incidence and prevalence of otosclerosis in the non-Caucasian population. Histopathologically normal endochondral bone of the otic capsule is replaced by disorganised foci of Haversian bone which ultimately becomes sclerotic and dense. Otospongiosis, the early or active phase of otosclerosis, is characterized by the presence of spongy irregular vascular foci of demineralised bone. This is followed by an otosclerotic or inactive phase where these diseased foci become less vascular, forming dense bone[4].
Otosclerosis can be divided into 2 types: Fenestral and retrofenestral, depending on the topography of the lesions. Fenestral lesions are in the lateral wall of the otic capsule, i.e., the regions of the round and oval windows, promontory, and tympanic segment of the fallopian canal. The retrofenesteral type affects the labyrinthine capsule, including the pericochlear region, the semicircular canals, internal acoustic meatus, vestibule, and cochlear and vestibular aqueducts[5,6].
Diagnosis is based on a combination of medical history, physical examination, audiological testing and imaging. The clinical findings include conductive, mixed, or rarely, pure sensorineural hearing loss and vertiginous symptoms in the absence of middle ear inflammation[2,7,8]. Surgical or histological confirmation is important in correlating clinical findings.
High resolution computed tomography (HRCT) is the gold standard imaging modality in the diagnosis of otosclerosis; it detects pathologic bone lesions in and around the stapes footplate, cochlea, and labyrinth[9-11]. In active otospongiosis, disease foci are visualised on CT as areas of reduced bone density and appear as increased bony radiolucency in the otic capsule, typically at the fissula ante fenestram, just anterior to the oval window in the fenestral type of the disease. HRCT can also demonstrate disease within the peri-labyrinthine bone and the cochlea in the retrofenestral subgroup. CT highlights differences in the density of the capsule’s outline, the so called double ring sign, which is a low density demineralised endochondral defect outlining the cochlea[7,9]. The density of disease foci increases in otosclerosis giving an appearance resembling normal otic capsule bone thereby complicating diagnosis and increasing the false negative rates[1,2,8].
HRCT may also be useful in distinguishing between otosclerosis and other pathological conditions such as tympanosclerosis, cholesteatoma, ossicular fixation and congenital malformations[9-11]. Its use in the preoperative stage for otosclerosis surgery remains debatable[12]. The aim of this study is to evaluate the sensitivity and specificity of HRCT in the diagnosis of otosclerosis using the best available evidence.
A contemporary literature review regarding the use of HRCT imaging in the diagnosis of otosclerosis was undertaken. A PubMed, MEDLINE and Google Scholar database search using terms “high resolution computed tomography”, “HRCT”, “CT”, “otosclerosis”, “diagnosis”, “sensitivity”, “specificity” in all combinations was completed. Abstracts were reviewed independently by two authors and relevant articles were then evaluated. Inclusion criteria were Level I-III studies where a diagnostic work up consisting of history and otolaryngology examination; speech/pure tone audiometry; tympanometry; and imaging in the form of HCRT had been carried out. We also included other studies where a CT diagnosis of otosclerosis was confirmed histologically in the absence of clinical information. Exclusion criteria were level IV-V evidence, studies where HRCT was used postoperatively and studies prior to 2000.
Level of evidence was assigned in accordance with the Oxford Centre for Evidence-based Medicine guidance, in a hierarchy of evidence strength from randomised controlled trials (level I), cohort studies (level II), case-control studies (level III), case series (level IV) to expert opinion and, case reports (level V) with suffixes “a” and “b” denoting a systematic review and an individual study respectively[13].
Statistical analysis was performed using MedCalc (Ostend, Belgium).
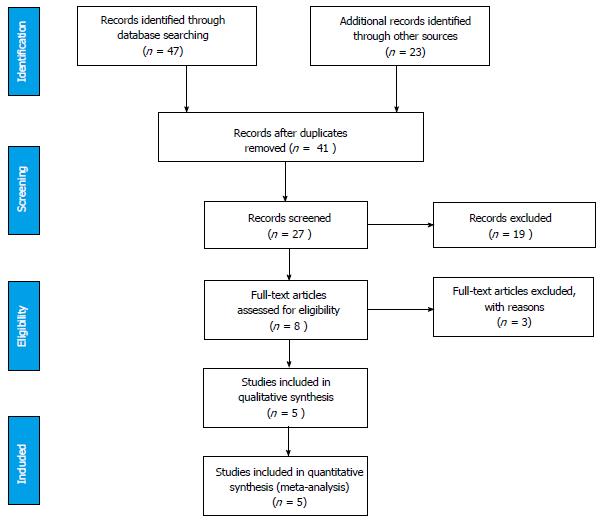
Figure 1 summarises the PRISMA systematic review flow diagram; the checklist is available as a supplementary file.
The 5 level III studies (Table 1) had a combined pool of 206 ears and 130 patients. A HRCT bone protocol was utilised in all studies. Axial and coronal reconstruction of the HRCT images with a slice thickness ranging from 0.6-2 mm were reviewed on a computerised picture archiving system and analysed in a blinded fashion by a radiologist, otologist or both. Otosclerotic foci were defined as hypodense lesions in the otic capsule or thickening/obliteration of the round and oval windows.
All analysed studies used control groups. The age and sex of the control group was not always included in the studies. There was a clear definition of control groups and we judged the risk of bias to be low, given that these included confirmed otosclerosis negative groups, vestibular schwannoma patients and contralateral ears in facial palsy patients.
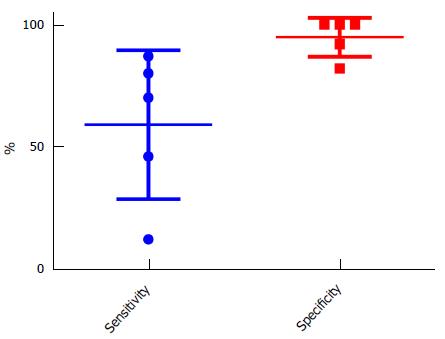
Quantitative synthesis analysis demonstrated low sensitivity of 58% (95%CI: 49.4-66.9), a high specificity of 95% (95%CI: 89.9-98.0) and a positive predictive value of 92% (95%CI: 84.1-95.8). Negative predictive value was 71% (95%CI: 66.1-74.7) (Figure 2). The majority of the otosclerotic foci identified on CT were in the fenestral region and a combination of fenestral and retrofenestral foci was second most common.
Quesnel et al[1]’s study demonstrated an excellent correlation between CT imaging on a series of temporal bones with otosclerosis and corresponding histology slides of the same. The same study also concluded that CT can diagnose endosteal margin involvement (63% sensitivity) but cannot be relied upon to exclude it[1].
Lee et al[2]’s study specifically focused on a specific ethnic group (Taiwanese) with the ultimate objective of elucidating the tomographic findings of otosclerosis in that group.
In Zhu et al[14]’s study all positive results had a double ring sign on HRCT. This study was the outlier by some distance and markedly affected the overall sensitivity of the pooled analysis. It is questionable that such a low diagnostic performance (4 of 34) should be possible and it is notable that some data was extrapolated as the primary aim of this study, like Zhu et al[14], was to assess the role of automated bone densitometry to diagnose otosclerosis. However, the study was retained within the analysis.
Quesnel et al[1] demonstrated a false positive rate of 8% (3/36) in their control group. These false positive cases which had appeared as hypodense lesions like otospongiotic foci were shown to be areas of increased connective tissue and vessels on histology. In another study a hypodense area in the anterior vestibule of 1 temporal bone in the control group was identified and judged to be a silent otosclerotic foci even though the subject was asymptomatic[8]. This was purely speculative and was not confirmed surgically.
A longer duration of disease was linked to multiple lesions in the otic capsule, false negative rates and larger preoperative air-bone gap (Vicente et al[8], 2006 and Lee et al[2], 2009).
In this review, we sought to elucidate the diagnostic performance of HRCT in otosclerosis using current level I-III studies. Numerous level IV and V papers have reported a wide-ranging sensitivity between 34% and 95%, with more recent studies suggesting values above 90%[10]. HRCT scans in otosclerosis are typically acquired using a bone algorithm with a slice thickness of 1 mm or less; slice thickness greater than 1 mm leads to increased false negative rates. Various studies have demonstrated that the sensitivity of HRCT is limited by inframillimetre and superficial foci, inactive disease, and density variations of less than 200 hounsfield units which are imperceptible to the naked eye[7,10].
The advent of improved CT scanning machines with improved collimation is thought to have raised the quality of the images available for analysis[1,2]. This, allied to the use of computerised workstations such as PACS for image analysis, has led to a higher diagnostic yield. Computerised workstations afford the ability to zoom in and scroll through images leading to a better appreciation of subtle abnormalities[16].
Overall, the analysed studies demonstrated a low sensitivity, high specificity and a high positive predictive value. However, there were wide confidence intervals particularly in the sensitivity, largely due to Zhu et al[14] study (12%). This study has limitations and has a particularly poor sensitivity in comparison to all other studies, possibly in part due to their primary aim being a densitometry study alongside with possible differing expertise levels. Other considerations include the disparate characteristics of the tested populations and the possible differences in the stages of the disease when the scans were performed. For instance, Lee et al[2]’s study (46% sensitivity) focused exclusively on a Taiwanese study group. With previous reports[17,18] suggesting a low sensitivity and incidence in other Asian ethnic groups it is impossible to ascertain whether the relatively low sensitivity in Lee et al[2]’s study is due to ethnic differences per se or is a manifestation of other factors such as patients presenting late in the otosclerotic phase. The rarity of the disease in Asians and the dearth of otologists with expertise in stapes surgery has led to a preference for non-surgical treatment amongst the greater proportion of patients with otosclerosis within the Taiwanese subgroup[2]. These factors are inevitably linked to the late presentation and arguably the low sensitivity on HRCT. Wider application of these findings is limited by the unique characteristics of the subgroup.
Quesnel et al[1] provide some useful insight into the relationship between HRCT and the size of disease foci. By matching presumed foci of otosclerosis identified on axial imaging with corresponding histology slides they demonstrate a sensitivity of 80%. The false negative results were due to the presence of an inframillimetres lesion which interestingly had not become clinically apparent. By correlating HRCT findings and histology, Quesnel et al[1] provide good evidence for the utility of HRCT in otosclerosis given that clinical/histopathological diagnosis is the gold standard in confirming pathology. Unfortunately their study is limited by a small sample size (18 ears) and the fact that the conditions under which the study was carried out are not easily reproducible clinically.
Our review shows that HRCT is better at identifying fenestral otospongiosis, thus confirming findings from previous studies. Identifying retrofenestral and endosteal margin involvement remains challenging notwithstanding that retrofenesteral otosclerosis is less common than fenesteral disease[8,10,14]. Studies have reported the limitations of HRCT in diagnosing retrofenestral otosclerosis with Dudau et al[19] suggesting a sensitivity of 58%. The main areas of interest in retrofenesteral otosclerosis are the cochlear, pericochlear, and the areas anterior to the round window niche[1,15]. Clinically, the presence of cochlear disease has implications for planning treatment and counselling patients because of the risk of developing sensorineural hearing loss. This makes preoperative diagnosis useful.
Unfortunately, CT diagnosis remains problematic particularly where otospongiotic foci are small and where other conditions that demineralise the otic capsule such as osteogenesis imperfecta, Paget’s disease or syphilis are considered[2,8]. These limitations are highlighted in Quesnel et al[1]’s study where CT had a sensitivity of 63% in identifying endosteal margin involvement.1The false negatives where due to inframillimetre disease. This illustrates that while HRCT can identify endosteal lesions it cannot be relied upon to conclusively rule it out.
Quesnel et al[1] and Vicente et al[8]’s studies identified abnormalities on HRCT in their respective control groups. Having dismissed findings of mild pericochlear lucency as a non-specific sign and therefore not necessarily suggestive of otosclerosis, Vicente et al[8] concluded that a hypodense focus anterior to the wall of the vestibule in one of the control ears was suggestive of silent otosclerosis. This taken in context with Quesnel et al[1]’s study where presumed area of otosclerotic foci on HRCT were shown to be areas of connective tissue and vessels on histology highlights the limitations of HRCT in diagnosing otosclerosis: Normal variants and other disease processes can appear as otosclerosis on HRCT[1,8]. Diagnosis of otosclerosis remains clinical and HRCT can play an ancillary role.
The studies included in our review used small sample sizes which makes them vulnerable to some of the limitations associated with such studies, i.e., underpowered, with large confidence intervals and heterogeneity. In addition, we have pooled data from disparate groups which adds to the limitations of using small samples. This, however, must be taken in the context of an overall dearth in studies that are level III or above whose primary aim is to investigate the utility of HRCT in diagnosing otosclerosis. Also, 2 of the 5 studies reviewed are retrospective and therefore prone to the shortcomings of such studies. Furthermore, because we have relied upon authors reporting of methodology and results for quality assessment and data extraction we cannot eliminate all bias.
The sensitivity and specificity of HRCT in diagnosing otosclerosis were not always the primary objective of all the studies included; in some studies, this was an indirect measure. For instance, in studies examining the utility of HRCT bone densitometry in otosclerosis (Grayeli et al[15] 2004 and Zhu et al[14] 2010). Zhu’s study in particular demonstrates an outlying sensitivity that had to be extrapolated from their study. Had this study been excluded the sensitivity of this pooled dataset would be 71%. It is unusual to have such a low diagnostic performance and this may reflect patient factors, disease factors (i.e., advanced disease) or local expertise factors. This demonstrates the inherent difficulty in pooling data from differing authors and studies and serves as a significant limiting factor in this analysis.
In conclusion, Based on current level III evidence HRCT has a high specificity and positive predictive value and a relatively low sensitivity in diagnosing otosclerosis. HRCT has a high sensitivity in identifying the more prevalent fenestral subtype of otosclerosis, particularly lesions in the fissula ante fenestram.
Inframillimetres lesions, retrofenestral lesions and dense sclerotic lesions present a diagnostic challenge despite the advent of more advanced CT scanners and better understanding of otosclerosis as a disease process. Diagnosis of otosclerosis remains clinical and HRCT can be a useful adjunct especially when assessing the extent of disease and when excluding other causes.
Otosclerosis is focal bone dyscrasia of unknown aetiology which predominantly affects only the endochondral bone of the otic capsule in humans. Patients typically present with conductive hearing loss. The diagnosis of otosclerosis is based on a combination of medical history, physical examination, audiological testing and imaging.
High resolution computed tomography (HRCT) of the temporal bones is the current imaging modality of choice in the investigation of otosclerosis. However, as demonstrated in this study and others, it has variable sensitivity and specificity.
This study highlights the value and limitations of HRCT in the diagnosis of Otosclerosis. Some studies in the literature are exploring the utility of cone beam computed tomography (CBCT) as an alternative to HRCT in the investigation of otosclerosis. However, these are in their infancy and time will tell whether CBCT supersedes HRCT as the modality of choice in imaging the middle ear.
HRCT is the gold standard imaging technique in investigating the middle ear. It may be useful in distinguishing between otosclerosis and other pathological conditions of the middle ear such as tympanosclerosis, cholesteatoma, ossicular fixation and congenital malformations
Otosclerosis is a bony dyscrasia of the inner ear otic capsule. HRCT has a significant role in imaging the labyrinthine and bony capsule of the temporal bone. The extent of otosclerosis into the cochlear capsule can be quantitatively evaluated using densitometric measurements. In this manuscript, the authors focused on the sensitivity and specificity of HRCT in the diagnosis of otosclerosis. This systematic review indicates that HRCT is a useful imaging method in diagnosis of otosclerosis [HRCT has a high specificity (98%) and low sensitivity (63%) in diagnosing otosclerosis], supported by level III evidence. This review has some significance for clinicians and researchers working.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amiri M, Tan XR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Quesnel AM, Moonis G, Appel J, O’Malley JT, Curtin HD, McKenna MJ. In response to letter to the editor: “Correlation of computed tomography with histopathology in otoslcerosis”, Quesnel et al. Otol Neurotol 2013; 34(1): 22-8. Otol Neurotol. 2013;34:1546-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Lee TL, Wang MC, Lirng JF, Liao WH, Yu EC, Shiao AS. High-resolution computed tomography in the diagnosis of otosclerosis in Taiwan. J Chin Med Assoc. 2009;72:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Schrauwen I, Van Camp G. The etiology of otosclerosis: a combination of genes and environment. Laryngoscope. 2010;120:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Goh JP, Chan LL, Tan TY. MRI of cochlear otosclerosis. Br J Radiol. 2002;75:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Valvassori GE. Imaging of otosclerosis. Otolaryngol Clin North Am. 1993;26:359-371. [PubMed] |
| 6. | Swartz JD, Faerber EN, Wolfson RJ, Marlowe FI. Fenestral otosclerosis: significance of preoperative CT evaluation. Radiology. 1984;151:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Lee TC, Aviv RI, Chen JM, Nedzelski JM, Fox AJ, Symons SP. CT grading of otosclerosis. AJNR Am J Neuroradiol. 2009;30:1435-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Vicente Ade O, Yamashita HK, Albernaz PL, Penido Nde O. Computed tomography in the diagnosis of otosclerosis. Otolaryngol Head Neck Surg. 2006;134:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Lagleyre S, Sorrentino T, Calmels MN, Shin YJ, Escudé B, Deguine O, Fraysse B. Reliability of high-resolution CT scan in diagnosis of otosclerosis. Otol Neurotol. 2009;30:1152-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Shin YJ, Fraysse B, Deguine O, Cognard C, Charlet JP, Sévely A. Sensorineural hearing loss and otosclerosis: a clinical and radiologic survey of 437 cases. Acta Otolaryngol. 2001;121:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Marx M, Lagleyre S, Escudé B, Demeslay J, Elhadi T, Deguine O, Fraysse B. Correlations between CT scan findings and hearing thresholds in otosclerosis. Acta Otolaryngol. 2011;131:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Ayache D, Lejeune D, Williams MT. Imaging of postoperative sensorineural complications of stapes surgery: a pictorial essay. Adv Otorhinolaryngol. 2007;65:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Howick J. Oxford Centre for Evidence-based Medicine - Levels of Evidence [Centre for Evidence-based Medicine website]. 2009;(Accessed August 26, 2012) Available from: http://www.cebm.net/index.aspx?o=1025. |
| 14. | Zhu MM, Sha Y, Zhuang PY, Olszewski AE, Jiang JQ, Xu JH, Xu CM, Chen B. Relationship between high-resolution computed tomography densitometry and audiometry in otosclerosis. Auris Nasus Larynx. 2010;37:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Grayeli AB, Yrieix CS, Imauchi Y, Cyna-gorse F, Ferrary E, Sterkers O. Temporal bone density measurements using CT in otosclerosis. Acta Oto-Laryngologica. 2004;124:1136-1140. |
| 16. | Huang TS, Lee FP. Surgically confirmed clinical otosclerosis among the Chinese. Arch Otolaryngol Head Neck Surg. 1988;114:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Joseph RB, Frazer JP. Otosclerosis incidence in caucasians and japanese. Arch Otolaryngol. 1964;80:256-262. [PubMed] |
| 18. | Wegner I, van Waes AM, Bittermann AJ, Buitinck SH, Dekker CF, Kurk SA, Rados M, Grolman W. A Systematic Review of the Diagnostic Value of CT Imaging in Diagnosing Otosclerosis. Otol Neurotol. 2016;37:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Dudau C, Salim F, Jiang D, Connor SE. Diagnostic efficacy and therapeutic impact of computed tomography in the evaluation of clinically suspected otosclerosis. Eur Radiol. 2017;27:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |