Published online Jul 16, 2017. doi: 10.12998/wjcc.v5.i7.264
Peer-review started: March 10, 2017
First decision: March 27, 2017
Revised: April 28, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: July 16, 2017
Processing time: 129 Days and 7.2 Hours
Celiac disease (CD) is a common and well defined autoimmune disorder caused by gliadin and related proteins of wheat, rye, and barley. Epidemiologic studies confirmed that CD is highly associated with other autoimmune diseases and with Down syndrome (DS). The symptomatic form of CD in patients with DS is more frequent than asymptomatic forms. However, growth impairment, anemia, intermittent diarrhea, and constipation are symptoms and signs typically of children with DS without CD. Late identification of the disease can lead to various complications, sometimes even very severe. Therefore, systematic screening for CD is essential in the management of children and adolescents with DS. Many medical organizations recommend screening in this group of patients. However, current policy statements vary in their recommendations for screening and there is still a need for establishing uniform diagnostic criteria.
Core tip: Celiac disease (CD) is more common in children with Down syndrome (DS) than in general population. Recommendations for screening for DS and CD remain controversial and we still lack standard evidence-based guidelines. This review, based on existing reports, indicates the need for establishing uniform and immediately applicable diagnostic criteria.
- Citation: Pavlovic M, Berenji K, Bukurov M. Screening of celiac disease in Down syndrome - Old and new dilemmas. World J Clin Cases 2017; 5(7): 264-269
- URL: https://www.wjgnet.com/2307-8960/full/v5/i7/264.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i7.264
Down syndrome (DS) is a chromosomal disorder caused by trisomy and other aberrations of chromosome 21[1]. This syndrome was first described by Langdon Down[2] in 1886, while in 1959, French pediatrician and geneticist. Lejeune published a paper in which he identified trisomy 21 as its genetic cause[3]. It occurs with a prevalence of 1:733, therefore representing the most common chromosomal disorder and one of the leading causes of mental retardation[4]. DS is characterized by many typical somatic and visceral malformations. People with this syndrome are prone to autoimmune and other diseases, such as hearing and vision problems, immune dysfunction, obstructive sleep apnea, Alzheimer disease-like dementia, megakaryoblastic leukemia, hypothyroidism, diabetes mellitus (DM), and celiac disease (CD)[1,4].
CD is an autoimmune disorder induced by gluten-containing food from seeds of wheat, rye, and barley[5]. It can develop at any age as a result of an inherited (polygenic) disposition and exposure to gluten. Research carried out during the last two decades has shown that a central role in the occurrence of the disease is played by MHC class II HLA antigens: HLA-DQ2 and HLA-DQ8[6]. The absence of HLA-DQ2 and HLA-DQ8 has a strong negative predictive value for CD[7]. Therefore, the question remains, why only a small percent of patients develops CD while approximately 40% of the population carries HLA-DQ2/DQ8 alleles and is exposed to gluten without developing a disease.
Even though enteropathy is the primary characteristic of the disease, CD may involve other extraintestinal organs[8]. Based on clinical, serological and histological variations, CD may be classified into two basic types: symptomatic and asymptomatic. Within symptomatic form of the disease, there are forms with classical and atypical clinical picture[9]. Classical form of CD occurs in infants and toddlers (9-36 mo) and it is characterized by gradual, rarely sudden onset of the disease. It is presented with a chronic diarrheal disorder, anorexia, vomiting, abdominal distension and pain, and in the most severe cases, with celiac crisis[10]. In the past two decades, the classical clinical manifestations in patients became less common, and we can see the emergence of a growing number of cases with the atypical form of CD (1:8 in general population)[11]. Among adolescents and adults, disease presents in atypical form, with absent or mild gastrointestinal symptoms, and with more common extraintestinal manifestations, such as sideropenic anemia resistant to oral therapy with iron, delay in longitudinal growth, marked thinness, chronic fatigue, osteopenia and osteoporosis, enamel hypoplasia, arthralgia, myalgia, epilepsy, ataxia, polyneuropathy, vitiligo, alopecia, dermatitis herpetiformis, etc[12].
Autoimmune diseases are ten times more common in patients with CD compared to general population. Such diseases include type 1 DM, autoimmune thyroid disease, Sjögren’s syndrome, Addison’s disease, chronic active hepatitis (elevated transaminases), primary biliary cirrhosis, IgA nephropathy, and juvenile chronic arthritis. Almost the same prevalence of the disease is also found in some chromosomal aberration disorders, such as Turner syndrome, Williams syndrome, and DS[13,14].
Diagnosis of CD is based on histological analysis of duodenal biopsies, HLA testing for HLA-DQ2 and HLA-DQ8, and detection of specific autoantibodies (mostly immunoglobulin A tissue transglutaminase - anti-TG2 and/or anti-endomysial antibodies - EMA). Gliadin antibody test and IgG class anti-TG2 antibody does not have the same specificity and clinical relevance. Use of a gluten-free diet is an effective treatment for CD as it has been shown to decrease the severity of clinical symptoms and reduce the risk of complications[6-8,15].
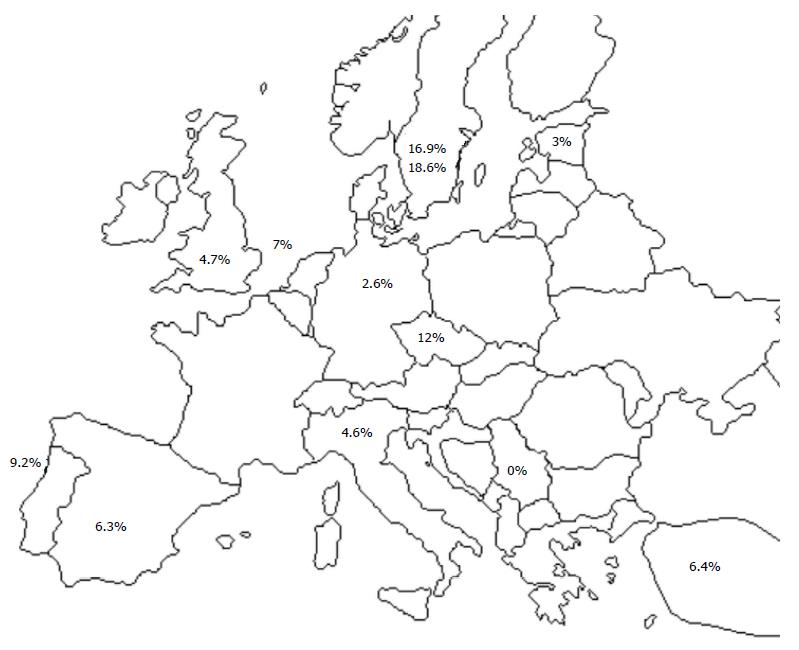
Association between CD and DS was first described in 1975, by Bentley et al[16]. Since then, many papers were published in Europe, reporting the prevalence of CD in DS patients to be from 0-18.6%[17-28] (Figure 1), which is far more prevalent than CD in general population (1% in Western countries)[29].
Similar prevalence of CD among DS patients were found in the United States - 3.8% and 10.3%[30,31], Australia 3.9%[32], Argentina - 3.6%[33], and Brazil - 5.6%[34]. The reported rates are probably overestimated because most of the authors did not perform small bowel biopsy in all DS patients with positive serology[20,23,25-27,31-34]. Variability in prevalence may have been caused by differences in type of antibody used for screening, different cohort sizes (25-1453), variable age stratification and applied criteria for CD diagnosis. Most of the studies were conducted on patients receiving care from local medical centers, and only a few studies were conducted at the level of the region or a country[18,20,24,32]. Furthermore, in the majority of children, the authors did not perform testing for HLA-DQ2 and HLA-DQ8 recommended by European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) criteria[15].
In their study, Pavlovic et al[17] did not find CD in any of 82 children with DS. This can be explained by age of children (8 mo to 8.6 years), but further serological monitoring of these children would probably show the presence of the disease in this group of patients. Higher prevalence was found only in two previous smaller series from Sweden; Jansson and Johansson[23] screened 65 DS patients and they found CD prevalence of 16.9% while Carlsson et al[24] reported the similar prevalence of 18.6% (8/43). These regional differences raise the question about the relationship of environmental factors and ethnic influence.
Compared to the general population, the symptomatic form of CD is more frequent in DS children than asymptomatic form[35]. However, about one-third of DS patients with CD have no gastrointestinal symptoms[20]. In children with DS and symptomatic form of CD, growth failure, anemia, intermittent diarrhea, vomiting, and constipation are described as the most common manifestations of the disease[36]. Their significance in clinical practice is not entirely obvious, bearing in mind the occurrence of the same symptoms and signs in children with DS, but without CD. Because of intellectual disability, DS patients may also be unable to accurately describe their symptoms. Growth failure as the manifestation of CD has little significance in children with DS, considering that the associated malformations, such as congenital heart defects and hypothyroidism, may also have the same effect[37]. Late identification or missed diagnosis of CD in DS patients can lead to failure to thrive, anemia, osteoporosis, and lymphoma[14,15].
The screening is planned on the basis of prevalence of the disease, sensitivity and specificity of tests, complications or comorbidities of the disease, effectiveness of the therapy (gluten-free diet), and costs. Swigonski et al[38] believe that the consistent use of serological screening for CD might not be entirely justified, primarily for economic reasons. Kolek et al[19] suggest CD screening only in patients with symptoms (loose stool, constipation, abdominal discomfort), but not in patients without symptoms. Mackey et al[39] suggested routine follow-up testing at least every 3 years for all children with DS, and yearly CD screening for patients who are serology positive and biopsy-negative.
Despite some common diseases associated with DS have clear screening guidelines, e.g., thyroid function, screening for CD remains controversial still lacking standard evidence-based guidelines (Table 1).
| Association | Screening for other diseases | CD screening | CD antibodies | Further CD antibodies testing | HLA testing |
| United Kingdom Down’s Syndrome Medical Interest Group[41] | Thyroid function | No | No | No | No |
| American Academy of Pediatrics[42] | Thyroid function, anemia | Symptomatic patients | IgA, IgA anti-TG2 | No | No |
| American Family Physician[43] | Thyroid function, diabetes mellitus | Not for adult | No | No | No |
| American Gastroenterological Association[44] | Symptomatic patients | IgA anti-TG2, IgA EMA | No | If other tests is not clear | |
| National Institute for Health and Care Excellence[45] | In all patients | IgA anti-TG2 | No | No | |
| European Down Syndrome Association[46] | Thyroid function, anemia, immunological defects | In all patients | IgG, IgA AGA, IgA anti-TG2, IgA EMA | Annually | No |
| Down’s Syndrome Medical Interest Group[47] | Thyroid function | At 2-3 yr in all patients | IgA EMA | No | No |
| North American Society for Pediatric Gastroenterology, Hepatology and Nutrition[48] | After 3 yr in all patients | IgA, IgA anti-TG2 | Some years | If IgA anti-TG2 negative | |
| European Society for Pediatric Gastroenterology, Hepatology and Nutrition[15] | After 2 yr in all patients | IgA anti-TG2 if HLA positive | Every 2 to 3 yr in DQ2 or DQ8 positive children | Yes |
The healthcare guidelines for patients with DS developed by the American Academy of Pediatrics (AAP)[40] published in 2001 and Down’s Syndrome Medical Interest Group United Kingdom and Ireland[41] did not made any recommendations for CD screening, even though they advised thyroid screening tests annually (risk of thyroid disease is 15%). Ten years later, AAP changed its position and advised screening for CD in the presence of symptoms, such as protracted constipation, slow growth, unexplained failure to thrive and anemia[42]. American Gastroenterological Association suggested that antibodies testing for CD is justified in patients with symptoms, but not those without symptoms and that HLA testing is appropriate only when the diagnosis based on other tests is not clear[44]. The recent recommendations by National Institute for Health and Care Excellence consider serological testing for CD in DS patients with IgA anti-TG2 as the first line of screening, and IgA EMA only if IgA anti-TG2 is weakly positive[45]. These recommendations do not imply HLA testing in the initial diagnosis of CD. The European Down Syndrome Association recommends blood tests for anemia, thyroid disease, CD and autoimmune disorders at 12 mo, and yearly thereafter, until old age in all patients[46]. The Down’s Syndrome Medical Interest group[47] recommends lifetime annual thyroid screening, and one-time screening for CD between ages 2 and 3, although North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) reports that the youngest child with both DS and CD diagnosed through screening was 3.2 years old. On the other hand, NASPGHAN has suggested that CD screening of children with DS once in a lifetime is not enough and that periodic screening should be done[48]. Finally, according to the latest recommendations of the ESPGHAN from 2012, human leukocyte antigen HLA-DQ2 and HLA-DQ8 typing should be the first line of screening[15]. In patients who are HLA-DQ2 and HLA-DQ8 negative, further serological testing is unnecessary. If the patient is DQ8 and/or DQ2 positive, then an anti-TG2 IgA test and total IgA determination should be performed. If antibodies are negative, repeated testing for CD-specific antibodies is recommended every 2 to 3 years. Although HLA typing is relatively expensive and not always feasible, finally, it likely seems to be a cost-effective procedure because the significant proportion of patients can be excluded from further antibodies testing.
DS patients have increased the risk of congenital malformations and a higher incidence of CD. Current evidence has important implications to support obligatory screening for CD in DS patients. Currently, many policy statements vary in their recommendations, and there is a need for further harmonization. The strategy should aim at early diagnosis and treatment of the condition in order to prevent the development of osteoporosis and lymphoma, as the most severe complication of this disease.
The authors wish to express their gratitude to Professor Nedeljko Radlovic for his assistance and support.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Serbia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Biltagi M, Dutta AK S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Patton MA. Genetics. Forfar & Arneil’s Textbook of Pediatrics. Edinburgh: Churchill Livingstone 2003; 407-441. |
| 2. | Down JL. Observations on an ethnic classification of idiots. 1866. Ment Retard. 1995;33:54-56. [PubMed] |
| 3. | Lejeune J, Gauthier M, Turpin R. Human chromosomes in tissue cultures. C R Hebd Seances Acad Sci. 1959;248:602-603. [PubMed] |
| 4. | Summar K, Lee B. Down syndrome and other abnormalities of chromosome number. Nelson textbook of pediatrics. Philadelphia: Saunders 2011; 394-404. |
| 5. | Mäki M, Lohi O. Celiac disease. Pediatric gastrointestinal disease. Hamilton: BC Decker Inc 2004; 933-943. |
| 6. | Ruiz-Ortiz E, Montraveta M, Cabré E, Herrero-Mata MJ, Pujol-Borrell R, Palou E, Faner R. HLA-DQ2/DQ8 and HLA-DQB1*02 homozygosity typing by real-time polymerase chain reaction for the assessment of celiac disease genetic risk: evaluation of a Spanish celiac population. Tissue Antigens. 2014;84:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Karell K, Louka AS, Moodie SJ, Ascher H, Clot F, Greco L, Ciclitira PJ, Sollid LM, Partanen J; European Genetics Cluster on Celiac Disease. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 412] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Polanco I. Celiac disease. J Pediatr Gastroenterol Nutr. 2008;47 Suppl 1:S3-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Radlovic N, Lekovic Z, Radlovic V, Simic D, Vuletic B, Ducic S, Stojsic Z. Celiac crisis in children in Serbia. Ital J Pediatr. 2016;42:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Rewers M. Epidemiology of celiac disease: what are the prevalence, incidence, and progression of celiac disease? Gastroenterology. 2005;128:S47-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 12. | Admou B, Essaadouni L, Krati K, Zaher K, Sbihi M, Chabaa L, Belaabidia B, Alaoui-Yazidi A. Atypical celiac disease: from recognizing to managing. Gastroenterol Res Pract. 2012;2012:637187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology. 2005;128:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Bai JC, Fried M, Corazza GR, Schuppan D, Farthing M, Catassi C, Greco L, Cohen H, Ciacci C, Eliakim R. World Gastroenterology Organisation global guidelines on celiac disease. J Clin Gastroenterol. 2013;47:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1837] [Article Influence: 141.3] [Reference Citation Analysis (5)] |
| 16. | Bentley D. A case of Down’s syndrome complicated by retinoblastoma and celiac disease. Pediatrics. 1975;56:131-133. [PubMed] |
| 17. | Pavlovic M, Radlovic N, Lekovic Z, Stojsic Z, Puleva K, Berenji K. When to screen children with Down syndrome for celiac disease? J Trop Pediatr. 2010;56:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Uibo O, Teesalu K, Metskula K, Reimand T, Saat R, Sillat T, Reimand K, Talvik T, Uibo R. Screening for celiac disease in Down’s syndrome patients revealed cases of subtotal villous atrophy without typical for celiac disease HLA-DQ and tissue transglutaminase antibodies. World J Gastroenterol. 2006;12:1430-1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Kolek A, Vospĕlová J, Hermanová Z, Santavá A, Tichý M. Occurrence of coeliac disease in children with Down’s syndrome in north Moravia, Czech Republic. Eur J Pediatr. 2003;162:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Bonamico M, Mariani P, Danesi HM, Crisogianni M, Failla P, Gemme G, Quartino AR, Giannotti A, Castro M, Balli F, Lecora M, Andria G, Guariso G, Gabrielli O, Catassi C, Lazzari R, Balocco NA, De Virgiliis S, Culasso F, Romano C; SIGEP (Italian Society of Pediatric Gastroenterology and Hepatology) and Medical Genetic Group. Prevalence and clinical picture of celiac disease in italian down syndrome patients: a multicenter study. J Pediatr Gastroenterol Nutr. 2001;33:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Carnicer J, Farré C, Varea V, Vilar P, Moreno J, Artigas J. Prevalence of coeliac disease in Down’s syndrome. Eur J Gastroenterol Hepatol. 2001;13:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Zubillaga P, Vitoria JC, Arrieta A, Echaniz P, Garcia-Masdevall MD. Down’s syndrome and celiac disease. J Pediatr Gastroenterol Nutr. 1993;16:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Jansson U, Johansson C. Down syndrome and celiac disease. J Pediatr Gastroenterol Nutr. 1995;21:443-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Carlsson A, Axelsson I, Borulf S, Bredberg A, Forslund M, Lindberg B, Sjöberg K, Ivarsson SA. Prevalence of IgA-antigliadin antibodies and IgA-antiendomysium antibodies related to celiac disease in children with Down syndrome. Pediatrics. 1998;101:272-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Cogulu O, Ozkinay F, Gunduz C, Cankaya T, Aydogdu S, Ozgenc F, Kutukculer N, Ozkinay C. Celiac disease in children with Down syndrome: importance of follow-up and serologic screening. Pediatr Int. 2003;45:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | George EK, Mearin ML, Bouquet J, von Blomberg BM, Stapel SO, van Elburg RM, de Graaf EA. High frequency of celiac disease in Down syndrome. J Pediatr. 1996;128:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Storm W. Prevalence and diagnostic significance of gliadin antibodies in children with Down syndrome. Eur J Pediatr. 1990;149:833-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancers and immune related diseases associated with Down’s syndrome: a record linkage study. Arch Dis Child. 2004;89:1014-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Dubé C, Rostom A, Sy R, Cranney A, Saloojee N, Garritty C, Sampson M, Zhang L, Yazdi F, Mamaladze V. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastroenterology. 2005;128:S57-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 30. | Pueschel SM, Romano C, Failla P, Barone C, Pettinato R, Castellano Chiodo A, Plumari DL. A prevalence study of celiac disease in persons with Down syndrome residing in the United States of America. Acta Paediatr. 1999;88:953-956. [PubMed] [DOI] [Full Text] |
| 31. | Book L, Hart A, Black J, Feolo M, Zone JJ, Neuhausen SL. Prevalence and clinical characteristics of celiac disease in Downs syndrome in a US study. Am J Med Genet. 2001;98:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Gale L, Wimalaratna H, Brotodiharjo A, Duggan JM. Down’s syndrome is strongly associated with coeliac disease. Gut. 1997;40:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Rumbo M, Chirdo FG, Ben R, Saldungaray I, Villalobos R. Evaluation of coeliac disease serological markers in Down syndrome patients. Dig Liver Dis. 2002;34:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Nisihara RM, Kotze LM, Utiyama SR, Oliveira NP, Fiedler PT, Messias-Reason IT. Celiac disease in children and adolescents with Down syndrome. J Pediatr (Rio J). 2005;81:373-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, Bianchi FB. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Szaflarska-Popławska A, Soroczyńska-Wrzyszcz A, Barg E, Józefczuk J, Korczowski B, Grzybowska-Chlebowczyk U, Więcek S, Cukrowska B. Assessment of coeliac disease prevalence in patients with Down syndrome in Poland - a multi-centre study. Prz Gastroenterol. 2016;11:41-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Myrelid A, Gustafsson J, Ollars B, Annerén G. Growth charts for Down’s syndrome from birth to 18 years of age. Arch Dis Child. 2002;87:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Swigonski NL, Kuhlenschmidt HL, Bull MJ, Corkins MR, Downs SM. Screening for celiac disease in asymptomatic children with Down syndrome: cost-effectiveness of preventing lymphoma. Pediatrics. 2006;118:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Mackey J, Treem WR, Worley G, Boney A, Hart P, Kishnani PS. Frequency of celiac disease in individuals with Down syndrome in the United States. Clin Pediatr (Phila). 2001;40:249-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | American Academy of Pediatrics. Committee on Genetics. American Academy of Pediatrics: Health supervision for children with Down syndrome. Pediatrics. 2001;107:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 41. | King K, O’Gorman C, Gallagher S. Thyroid dysfunction in children with Down syndrome: a literature review. Ir J Med Sci. 2014;183:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Bull MJ. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 669] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 43. | Smith DS. Health care management of adults with Down syndrome. Am Fam Physician. 2001;64:1031-1038. [PubMed] |
| 44. | AGA Institute. AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977-1980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | National Institute for Health and Care Excellence. Coeliac disease: recognition, assessment and management. Internal Clinical Guidelines Team. NICE Guideline 2015, No. 20. Available from: http//www.nice.org.uk/guidance/ng20/chapter/Recommendations#referral-of-people-with-suspected-coeliac-disease. |
| 46. | European Down Syndrome Association (EDSA). Health care guidelines for people with Down syndrome, 2006. Available from: http//www.edsa.eu/wpcontent/uploads/2015/06/edsa_essentials_2_healthcare.pdf. |
| 47. | Cohen W. Health care guidelines for individuals with Down syndrome: 1999 revision. Down Synd. 1999;Q 4:1-15 Available from: http//www.ds-health.com/health99.htm. |
| 48. | Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1-19. [PubMed] |