Published online Jun 16, 2017. doi: 10.12998/wjcc.v5.i6.191
Peer-review started: January 19, 2017
First decision: March 8, 2017
Revised: March 21, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: June 16, 2017
Processing time: 147 Days and 17.9 Hours
The etiologic diagnosis of cerebrovascular diseases requires non-routine complementary examinations to be performed. Thus, in specific cases, after neuroimaging (computed tomography/magnetic resonance imaging cerebral scan sequences) and neurosonology (Doppler test of the supra-aortic trunks, transcranial echography and echocardiography), which academically allow us to classify the patients according to their etiologic stroke subtype, further examinations must be used to make a correct etiologic diagnostic. The present review aims to update knowledge about the usefulness of the different tests of blood and urine, plain chest radiography, X-ray of the spine, skull and abdomen, lumbar puncture, electroencephalography, evoked potentials, polysomnography, and pathologic examination after biopsy of the artery, skin, muscles, nerves, meninges, and brain, in the management of patients who have suffered an acute stroke.
Core tip: In selected cases of acute stroke, some complementary examinations (different from neuroimaging, neurosonology and cardiac tests) are needed for the adequate etiological diagnosis. For example, the polysomnographic study allows for the diagnosis of respiratory sleep disorders; urinalysis may rule out the presence of toxins related to stroke; the analysis of the cerebrospinal fluid eliminates the possibility of an infection or an inflammatory process of the central nervous system and the artery biopsy lets you diagnose inflammatory arteritis. The knowledge of the diagnostic performance of these complementary examinations, which are sometimes true diagnostic tests, is very useful in the daily clinical practice of stroke patients.
- Citation: Arboix A, Obach V, Sánchez MJ, Massons J. Complementary examinations other than neuroimaging and neurosonology in acute stroke. World J Clin Cases 2017; 5(6): 191-202
- URL: https://www.wjgnet.com/2307-8960/full/v5/i6/191.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i6.191
In patients presenting with acute stroke, a thorough medical history and complete neurological and physical examination should be performed, in order to initiate the diagnostic process. This diagnostic work includes syndromic diagnosis, nosological, etiological and pathophysiological background, and differential diagnosis.
Complementary examinations are all those explorations requested by the clinician or specialist providing care for the stroke patient. These examinations, rarely performed by the clinician, require more or less sophisticated tools which in turn demand highly specialization in their use or interpretation.
The prescription of a complementary examination in the management of acute stroke must comply, on the part of the clinician requesting it, a series of conditions: (1) know what possible information about diagnostic or therapy will provide us, and therefore, which are its indications. This information is fundamental because complementary examinations should only be requested when their results had the possibility to modify the diagnostic or therapeutic attitude towards the patient; (2) know the sensitivity and specificity of the requested examination. Sensitivity or positive predictive value is the probability of obtaining a positive result in a person with the disease under study. The specificity or negative predictive value is the probability of obtaining a negative result in a person who does not have the disease under study; (3) know the right time to order; (4) know the possible contraindications; and (5) inform the patient about the exploration and why it is necessary, obtaining the informed consent.
In this review we will examine the following complementary explorations: (1) blood biochemistry: general, toxicology, endocrinological study, immunological study; (2) microbiology and serology; (3) genetic study; (4) urine test; (5) lumbar puncture: cerebrospinal fluid; (6) plain radiography: thorax, spine, skull, abdomen; (7) electroencephalogram; (8) evoked potentials; (9) polysomnography; (10) neuropsychological study; and (11) pathological study: Biopsies: Artery, skin, muscular, nerve, meningeal, and cerebral.
Neuroimaging, neurosonology and hematological explorations are not analyzed in this review.
These tests are useful in patient requiring intravenous fluid therapy. They are also necessary to diagnose hypokalemia secondary to diuretic therapy. Hyponatremia may cause fluctuating focal neurological deficits, and simulate stroke or transient ischemic attacks. The cause of these neurological deficits is unclear, but it has been suggested that such metabolic disorders could unmask previously existing areas of clinically silent cerebral ischemia. In a recent clinical study hyponatremia occurred in 43% of stroke patients, 6% had hypernatremia, and 4% had both. Cerebral salt wasting was the most common cause of hyponatremia in stroke patients. Exceptionally, hypoparathyroidism and hyperparathyroidism, by variations of calcemia, can cause focal neurological deficits similar to those of stroke. True cerebral infarcts due to hypercalcemia secondary to persistent arterial vasospasm have also been described.
Glucose and glycosylated hemoglobin levels are the primary indicators of effective metabolic control of those patients diagnosed with diabetes. An analysis of glucose is required for the diagnosis of diabetes mellitus, one of the most common cerebrovascular risk factors in all types of stroke, but especially in lacunar and atherothrombotic infarctions.
Hyperglycemia in the onset of cerebrovascular diseases may cause larger lesions and a worse prognosis due to its harmful effect on the area of ischemic penumbra. Irrespective of other adverse prognostic factors - including advanced age, type and severity of stroke, and irreversible neurological deficit, hyperglycemia above 8 mmol/L is associated with an increased mortality and morbidity after acute stroke. Hyperglycemia may also be the answer to a serious brain injury. Decompensated hyperglycemic hyperosmolarity may cause a reduced cerebral flow, leading to focal epilepsy and even cerebral ischemia.
Likewise, in 2.4% of cases hypoglycemia may produce diverse clinical features, ranging from hemiparesis to coma, mimicking and, exceptionally, leading to cerebral infarction. The underlying pathophysiological mechanism is not well known, but has been ascribed to a selective neuronal vulnerability, to cerebral arterial spasms, or to a clinically silent cerebrovascular disease exposed by the hypoglycemia itself. Therefore, hypoglycemic hemiplegia is exceptional, although it should be borne in mind in diabetic patients with cerebral infarction treated with either insulin or oral hypoglycemic agents. In addition, hypoglycemia and hypoglycemic hemiplegia can result in insulinoma. Hypoglycemia may also result in permanent neurological damage if prolonged, caused by hypoglycemic necrosis usually affecting caudate nucleus, putamen, hippocampus and periventricular level. Finally, hypoglycemia is also a rare cause of non-valvular atrial fibrillation which is self-limited and reverts to sinus rhythm when managing this metabolic disorder.
It’s a valuable screening test in evaluating kidney function. Normal serum creatinine levels allow us ruling out renal failure while, on the other hand, increased levels act as a marker for generalized vascular disease. In patients with cerebral infarction, the presence of renal dysfunction is usually indicative of the repercussion of arteriosclerotic vascular disease on this target organ, or even acts a marker of the severity or duration of arterial hypertension, the main cerebrovascular risk factor.
Hypercholesterolemia is a risk factor for cerebral infarction, and may be a manifestation of thyroid disease, hepatic or pancreatic dysfunction, diabetes, and nephrotic syndrome. Hypertriglyceridemia (levels greater than 150 mg/dL or 1.7 mmol/L) is also a risk factor for coronary heart disease. Hyperlipidemias may be graded according to the Fredrickson classification, which is based on the pattern of lipoproteins on electrophoresis. Cholesterol associated with high-density lipoprotein (HDL-cholesterol) plays a protective role in atherosclerosis. In contrast, cholesterol linked to low density lipoproteins (LDL-cholesterol) favors the atherogenic process. The risk of cerebral infarction is greater in patients with low levels of HDL and elevated levels of LDL-cholesterol: A decreased HDL/LDL or HDL ratio per 100/total cholesterol.
Recent studies have demonstrated the protective effect of statins, specifically on atherosclerotic plaques regression, and its role as secondary prevention of cerebral infarction in patients with ischemic heart disease regardless of cholesterol figures. It is recommended to maintain the following levels of cholesterol: LDL < 50 mg/dL (2.59 mmol/L), HDL > 35 mg/dL (0.91 mmol/L), total cholesterol < 200 mg/dL and triglycerides < 200 mg/dL (2.26 mmol/L).
A protein alteration may suggest the presence of a hyperviscosity syndrome and guide physicians in the diagnosis of systemic diseases causing cerebrovascular pathology, such as collagen diseases, multiple myeloma, and Waldenstrom’s macroglobulinemia.
For its part, malnutrition is associated to increased risk for medical complications and mortality, mainly related to a high risk of hospital acquired infections (respiratory and urinary), decubitus ulcers, and longer hospital stay; all this is possibly due to an alteration in the immune function.
Furthermore, it has recently been reported that serum albumin levels below 4.2 g/dL are associated with increased mortality in patients with cerebral infarction.
The role of hyperuricemia and its possible association with coronary, peripheral, and cerebral arteriosclerosis is uncertain. Therefore, its relation to the pathogenesis of cerebral vascular disease is controversial. However, a recent study showed that intravenous administration of uric acid may have a protective effect on cerebral infarction since it appears to be effective in improving the clinical prognosis of patients, possibly because of its antioxidant properties.
They may be helpful in the differential diagnosis between a metabolic coma and a coma secondary to acute cerebral vascular disease, and also in the therapeutic management of patients with severe stroke and renal, pulmonary or cardiac complications.
A toxicological study may be necessary in the diagnostic evaluation of the unconscious patient. It may assist in atypical intracranial hemorrhages, in subarachnoid hemorrhages, and in identifying intoxicated patients with amphetamines, sympathomimetics or cocaine, which can cause hemorrhagic and ischemic strokes.
The determination of T3, T4 and TSH may be helpful in young patients with non-valvular atrial fibrillation and cardioembolic cerebral infarction, as well as in all patients with suspected hyperfunction or hypofunction of the thyroid gland.
Immunological studies (complement consumption, ANA, ENA, anti-DNA antibodies, rheumatoid factor, LE cells, cryoglobulins, circulating immune complexes, ANCA, mainly) should be performed in selected patients when regular diagnostic tests do not confirm the diagnosis of stroke subtype.
Most autoimmune or connective diseases, as systemic lupus erythematosus or vasculitis, may lead to TIA or stroke, in both arterial or venous thrombosis as well as subarachnoid or intracerebral hemorrhage, those due to the rupture of the affected cerebral vessel. As well, in addition to inflammatory arteritis, the arterial hypertension due to renal failure or opportunistic infections caused by immunosuppressive therapy may also be the cause of stroke or hemorrhage in those patients.
Table 1 shows the major antinuclear antibodies associated with connective tissue diseases.
| Antinuclear antibodies | |
| Native Anti-DNA | SLE |
| Anti-histone | Drug-induced SLE/SLE/RA/juvenile chronic arthritis |
| Anti-RNP | Mixed connective-tissue disease/SLE |
| Anti Sm | SLE |
| Anti-Ro/SS-A | Sjögren syndrome/SLE/neonatal lupus Subacute cutaneous SLE/SLE related to component deficiency |
| Anti-La/SS-B | Sjögren syndrome/SLE |
| Anti-Scl-70 | Diffuse scleroderma |
| Anticentromere | Scleroderma (CREST syndrome) |
| Anti-Jo1 | Polymyositis with interstitial pulmonary disease |
| Antinucleolar | Scleroderma |
Basal levels of lactic acid measurements are necessary if mitochondrial disease is suspected. Since recently, hyperhomocysteinemia proved to be a cerebrovascular risk factor, homocysteine testing should be requested if a patient if suspected of having hyperhomocysteinemia or in patients with essential lacunar or non-lacunar cerebral ischemia.
Table 2 shows hematological and biochemical laboratory parameters that contraindicate the administration of intravenous thrombolytic treatment in the acute phase of cerebral ischemia.
| Platelet count < 100000 |
| Glycaemia < 50 and/or > 400 mg/dL |
| Severe liver failure |
| Oral anticoagulants with INR > 1.7 |
| Heparin treatment and ATTP > 1.5 |
| Analytical parameters suspicious of acute pancreatitis |
Infections of the brain may be one of the etiologies of cerebrovascular disease. In a recent study, brain infections accounted for 15.7% of the consecutive strokes of unusual etiology admitted to a neurology department over a 10-year period. Stroke or transient ischemic attack can be caused primarily by: Infectious arteritis, chronic meningitis, acute bacterial meningitis, viruses, certain helminthiasis, cat scratch disease, carotid inflammation resulting from contiguous pharyngitis, tonsillitis or lymphadenitis, and infective endocarditis.
Bacterial, viral, fungal infections (mainly Cryptococcus, Candida, Aspergillus or Mucor) or protozoa may affect the central nervous system and result in cerebrovascular disease, whose definitive etiological diagnosis requires special culture media.
In the Mediterranean area, cerebrovascular disease may be due to brucellosis, Mediterranean red button fever, mycoplasma infection, neuroborreliosis or herpes zoster infection, which is capable of causing periarterial inflammation with concomitant thrombosis. In some cases, bacterial meningitis, tuberculosis, leptospirosis, malaria and certain helminthiases (neurotrichinosis, cysticercosis and hydatidosis) may also cause cerebrovascular disease. In addition, stroke may be the presenting feature of the acquired immunodeficiency syndrome, and may be secondary to an opportunistic infection of the central nervous system. Cerebral embolism is the major neurological complication of infective endocarditis, and clinical examination provides a presumptive diagnosis subsequently confirmed by the identification of the germ in serial blood cultures.
The meningovascular lues should be included in the differential etiological diagnosis of acute cerebrovascular disease. Two general types are available for testing for syphilis: Reaginic and treponemal. The former detect nonspecific antibodies directed against treponemal lipid antigens, and the most used are venereal disease research laboratory (VDRL) and rapid plasma regain. Treponemal tests, for their part, detect antibodies that specifically target Treponema, and the most common are: TPI (Treponema immobilization test), FTA-ABS (test of fluorescent antibodies absorbed against Treponema) and MHA-TP (Treponema microhemagglutination). Non-specific tests can produce false positives, but not the treponemal, although they will remain positive throughout life.
Because meningovascular syphilis continues to be “the great imitator”, some authors recommend the routine practice of serological tests for syphilis in all patients with acute stroke. Other authors, however, suggest restrict them to those patients with cerebrovascular disease and absence of risk factors, high risk of seropositivity or atypical clinical course. Although positivity of serological tests for syphilis in the peripheral blood indicates the presence of meningovascular lues, positive cerebrospinal fluid is essential to provide definitive etiological confirmation, thus making it a highly specific diagnostic marker for the disease.
The genetic testing should be considered in those patients who present evidence of a suspected genetic condition of the cerebrovascular disease. Consideration will be given to the study of clotting disorders that can cause thrombophilia (protein S and protein deficiency, Factor V Leyden mutation and prothrombin gene mutation).
Eligible patients for genetic testing should have: (1) younger age than usual (< 50 years old); (2) family history (most especially affecting first-degree relatives and at younger ages) of ischemic heart disease, pulmonary thromboembolism/deep venous thrombosis, recurrent pregnancy loss, and peripheral arteriopathy; (3) absence of cardiovascular risk factors (or risk factors unrelated to cerebrovascular disease); (4) complete cardiovascular study without evidences that can explain cerebrovascular disease; (5) concomitant neurological entities (epilepsy in MELAS syndrome, dementia and migraine in CADASIL); (6) concomitant non-neurological diseases (ischemic heart disease, pulmonary thromboembolism/deep venous thrombosis, pregnancy loss, peripheral arteriopathy); (7) atypical radiological pattern (in MELAS syndrome) or multiple silent ischemic lesions in young adult (CADASIL, Fabry disease); (8) characteristic morphologic phenotype (in Marfan syndrome, Ehlers-Danlos); (9) skin lesions (angiokeratoma in Fabry disease, telangiectasia in Rendu-Osler-Weber disease); and (10) alterations in coagulation times (in hereditary hemostatic disorders).
Table 3 shows the main genetic disorders associated with ischemic stroke and Table 4 those related to hemorrhagic strokes.
| Coagulation related genes | Genetic pattern | Inheritance | Gene |
| Congenital deficiencies of clotting factors | |||
| Antithrombin III | Monogenic | AD | 1q23-25 |
| Protein C | Monogenic | AD/AR | 2q13-14 |
| Protein S | Monogenic | AD | 3p11.1-q11.2 |
| Heparin cofactor II | Monogenic | AD | 22q11 |
| Factor VII | Monogenic | AR | 13q34 |
| Factor XII | Monogenic | AR | 5q33-ter |
| Elevated factor VIII | Monogenic | ? | Xq28 |
| Plasminogen | Monogenic | AD | 6p26 |
| Plasminogen activators | Monogenic | AD | 8p12 |
| Polymorphism of clotting factors | |||
| Factor V leiden (G1619A) | Polymorphism | Mutation increases risk | 1q23 |
| Prothrombin G20210A | Polymorphism | Mutation increases risk | 11p11q12 |
| Sickle-cell disease | Monogenic | AR | Mutation A→T, Glu6Val in beta chain of hemoglobin 11p15.5 |
| Connective tissue disorders | |||
| Ehlers-Danlos type IV syndrome | Monogenic (genetic heterogeneity) | AD | Mutations Collagen gene type III (COL3·A1) 2q31 |
| Marfan syndrome | Polygenic | AD | Gene fibrillin-1 15q21.1 |
| AD | 3p24.2-p25 | ||
| Pseudoxanthoma elasticum | Polygenic | AR & AD | 16p13.1? |
| Neurofibromatosis type I | Monogenic (genetic heterogeneity) | AD | 17q11.2 |
| Tuberous sclerosis | Polygenic | AD | TSC1 9q34 |
| AD | TSC2 16p13 | ||
| AD | TSC3 and TSC4 ? | ||
| Vasculopathies | |||
| Fibromuscular dysplasia | Polygenic? | AD? | ? |
| Moya-moya disease | Polygenic | AD/AR? | 3p24.2-p26 |
| CADASIL | Monogenic | AD/AR? | 17q25 |
| AD | Notch3, 19p12 | ||
| Metabolic diseases | |||
| Homocystinuria | Monogenic (genetic heterogeneity) | AR | More frequent Cystathionine-beta-synthase 21q22.3 |
| Methylenetetrahydrofolate reductase | Monogenic | AR | 1p36.3 |
| Fabry disease | Monogenic | X-link R | GLA Xq21.3-22 |
| MELAS | mitochondrial | ||
| Genes and diabetes mellitus, arterial hypertension, dyslipidemia | Variable (genetic heterogeneity) | ||
| Genes and myocardiopathy, myxoma and familial arrhythmia | Variable (genetic heterogeneity) |
| Coagulation | Genetic pattern | Inheritance | Gene |
| Congenital deficiencies of clotting factors | |||
| Factor VIII | Monogenic | X-link R | Xq28 |
| Factor IX | Monogenic | X-link R | Xq27.1-q27.2 |
| Factor XIII | Monogenic | AR | 6p25-p24 |
| Factor VII | Monogenic | AR | 13q34 |
| Factor X | Monogenic | AR | 3q34 |
| Factor XI | Monogenic | AR | 4q35 |
| Afibrinogenemia | Monogenic | AR | 4q28 |
| Polymorphism of clotting factors | 1q23 | ||
| Factor V Leiden (G1619A) | Polymorphism | ||
| Factor XIII Val34Leu | Polymorphism | 6p25-p24 | |
| Factor XIII Tyr204Phe | Polymorphism | 6p25-p24 | |
| Factor XIII Pro564Leu | Polymorphism | 6p25-p24 | |
| Factor VII-323Del/Ins | Polymorphism | 13q34 | |
| PAI-I 4G/5G | Polymorphism | 7q21.3-q22 | |
| Platelet disorders | |||
| Thrombocytopenia-absent radius | Monogenic | AR | ? |
| Wiskott-Aldrich syndrome | Monogenic | X-link R | Xp11.23-p11.22 |
| Bernard-Soulier syndrome | Monogenic | AD | 22p11.2-17pter-p12 |
| Glanzmann thrombasthenia | Monogenic | AR | 17q21.32 |
| Storage pool deficiency | Genetic heterogeneity | ||
| Sickle-cell disease | Monogenic | AR | Mutation A→T, Glu6Val in beta chain of hemoglobin 11p15.5 |
| Vascular malformations | |||
| Multiple cavernomatosis | Polygenic | ||
| CCM1 | AD | 7q11.2-q21 | |
| CCM2 | AD | 7p15-13 | |
| CCM3 | AD | 3q25.2-27 | |
| Arteriovenous malformations | ? | ? | ? |
| Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber) | Polygenic | ||
| THH type 1 | AD | Endoglin gene, 9q | |
| THH type 2 | AD | Activin receptor-likekinase, 12q | |
| Von Hippel-Lindau disease | Monogenic | AD | 3p26-p25 |
| Bannayan-Zonana syndrome | Monogenic | AD | 10q23.3 |
| Familial venous malformations | Monogenic | AD | Mutation gene Tie-2, 9p |
| Cerebral aneurysms and SAH | Polygenic | ? | Ligament 5q22-q31 |
| Ligament 7q11 | |||
| Ligament 14q22 | |||
| Alpha-1 antitrypsin | Polymorphism | Alleles Z and S, 14q32.1 | |
| Endoglin gene | Polymorphism | Intron insertion 7, 9q | |
| MMP-9 gene | Polymorphism | -736 (CA)23 9q34.1 | |
| Connective tissue disorders | |||
| Ehlers-Danlos type IV syndrome | Monogenic (genetic heterogeneity) | AD | Mutations Collagen gene type III (COL3·A1) 2q31 |
| Marfan syndrome | Polygenic | AD | Gene fibrillin-1 15q21.1 |
| Polygenic | AD | 3p24.2-p25 | |
| Polycystic kidney disease | |||
| ADPKD 1 | AD | 16p13.3 | |
| ADPKD 2 | AD | 4q13-23 | |
| ADPKD 3 | AD | ? | |
| ARPKD | AR | 6p21.1-p12 | |
| Pseudoxanthoma elasticum | Polygenic | AR & AD | 16p13.1? |
| Neurofibromatosis type I | Monogenic (genetic heterogeneity) | AD | 17q11.2 |
| Tuberous sclerosis | Polygenic | AD | TSC1 9q34 |
| AD | TSC2 16p13 | ||
| AD | TSC3 and TSC4? | ||
| Vasculopathies | |||
| Fibromuscular dysplasia | Polygenic? | AD? | ? |
| Moya-moya disease | Polygenic | AD/AR? | 3p24.2-p26 |
| AD/AR? | 17q25 | ||
| CADASIL | Monogenic | AD | Notch3, 19p12 |
| Metabolic disorders | |||
| Fabry disease | Monogenic | X-link R | GLA Xq21.3-22 |
| MELAS | mitochondrial | ||
| Amyloidosis related genes | |||
| Hereditary cerebral hemorrhage with amyloidosis | |||
| Dutch type | Monogenic (genetic heterogeneity) | AD | Mutations amyloid-beta precursos protein, 21q21 |
| Icelandic type | Monogenic | AD | Substitution Leu68 → GlnCystatin C gene, 20p11.2 |
| Cerebral amyloid angiopathy | ? | ? | APOE, alleles E2, E4 |
| 19q13.2 | |||
| Transtiretine gene | Monogenic | AD | 18q11.2-q12.1 |
| Genes and HTA | Polygenic |
In patients with cerebrovascular disease is recommended to perform a biochemical analysis of urine.
Albumin: The presence of albumin may indicate nephropathy, and also Bence Jones proteinuria which may cause hyperviscosity syndrome. Albumin is usually associated with nephritic syndrome that can lead to a prothrombotic state.
Catecholamines and metabolites: Tests of catecholamines and their metabolites (free urinary catecholamines, urine metanephrines and vanillylmandelic acid) in 24-h urine may be useful in case of suspected hypertensive emergency secondary to pheochromocytoma.
Cyanide-nitroprusside test: It is useful to detect homocystinuria, an autosomal recessive disease due to deficiency of the cystathionine-synthase enzyme.
Toxicological study: Urine drug testing may be useful to detect the presence of drugs in the urine (cocaine, amphetamines, and sympathomimetics) and may be considered in young individuals with stroke who do not present known vascular risk factors.
Organic acids: Its determination can alert to the possibility of cerebrovascular disease related to metabolic disorders (organic acidemia of juvenile presentation, such as methylmalonic acidemia or Leigh’s disease).
They should be conducted in patients with vascular disease and febrile syndrome. Because urinary tract infections occur in 8%-14% of stroke patients admitted to hospital, they are the most common non-neurological medical complications. The presence of microhematuria can indicate concomitant renal infarction and guide the diagnosis of cerebral embolism of cardiac origin.
The cerebrospinal fluid (CSF) is obtained by doing a lumbar puncture (LP) to analyze its color (must be transparent, as rock water), pressure (15-20 cm H2O), protein (15-50 mg/100 mL), glucose (2.2-4.4 mmol/L) (50-75 mg/100 mL), cells (0-5 mononuclear cells/mm3), determination of the VDRL test, FTA-Abs test, and bacteriological study. It has been one of the most useful explorations in the diagnosis of patients with cerebrovascular disease for decades now.
LP is contraindicated by the presence of symptoms and signs suggestive of intracranial hypertension, of risk or evidence of cerebellar tonsillar herniation, of severe thrombopenia, and in the case of administration of anticoagulants.
The widespread availability of modern computed tomography (CT) and magnetic resonance imaging systems has significantly changed the diagnostic indications of LP to the extent that LP cannot be performed if there are no previous neuroimaging tests.
The current recommendations of LP in cerebrovascular pathology are the following[17,24,36-39]: (1) in case of “febrile syndrome” of unknown etiology, in order to rule out a meningeal or encephalic infectious process; (2) in patients diagnosed with or suspected of having an “infectious disease” affecting the central nervous system, such as Lyme disease, neurobrucellosis or neurocysticercosis; (3) patients with “constitutional syndrome” (asthenia, anorexia, weight loss) of unknown etiology, in order to rule out the existence of an infectious or neoplastic process; (4) positive “luetic serology”, in order to perform the syphilis test (VDRL) in CSF and confirm the meningovascular syphilis; (5) in patients diagnosed with or suspected of having “autoimmune disease”, such as isolated angiitis of the central nervous system, granulomatous angiitis, systemic vasculitis (polyarteritis nodosa, systemic lupus erythematosus, giant cell arteritis); and (6) clinical suspicion of “subarachnoid hemorrhage” when CT is negative or equivocal.
When a LP is performed and hematic CSF is detected, subarachnoid hemorrhage should be distinguished from traumatic LP. In order to differentiate them, a sample of CSF is collected in three consecutive tubes and, after centrifugation, examined with spectrophotometry - not visible to the naked eye - for the presence of xanthochromic supernatant. The presence of xanthochromia indicates subarachnoid hemorrhage. CSF xanthochromia may be observed within two weeks after hemorrhage.
Chest X-ray should be performed in all patients diagnosed with or suspected of having a cerebrovascular disease. The purpose of the test is obtaining information about the presence of possible pulmonary pathology (infectious or neoplastic), and heart disease mainly in relation to cardiac size (left ventricular hypertrophy) and aortic morphology.
Plain skull X-ray may be useful in previous head-injured patients to assess the presence of fissure or fracture of the cranial bones, especially the temporal squamous bone. It can provide additional information on etiological or concurrent pathologies of the patients’ neurological process, such as the dilation of the Turkish chair in pituitary tumors, and images of lysis or bone condensation in metastases or in Paget’s disease.
Radiological evaluation of the cervical spine is indicated in patients who present transient ischemic attacks related to the vertebrobasilar territory, and most especially in those episodes of neurological deficit triggered by movements of rotation or cephalic extension. The goal is identify the presence of uncoarthrosis, trigger of vertebral artery compression in the intravertebral trajectory (C6-C2).
In patients with cerebrovascular disease and arterial hypertension or intermittent claudication, abdominal X-ray allows accurate assessment of aneurysmal dilatation or calcification of the abdominal aorta.
The electroencephalogram (EEG) is the recording of electrical activity of the brain through electrodes placed on the surface of the skull. Currently, the EEG indications in patients with cerebrovascular disease are the following: (1) assisting with diagnosis of epileptic seizures in patients with transient alterations of consciousness secondary to previous or current cerebrovascular lesions; (2) evaluation of coma or pseudocoma states (alpha coma); (3) monitoring of brain function in carotid surgery (endarterectomy); and (4) clinical diagnosis of brain death.
Evoked potentials (EP) are the electrical manifestations generated by the brain in response to an external stimulus. Depending on the nature of the stimulus pattern, the evoked potential can be auditory, somatosensory, or visual.
The purpose is to determine whether or not a sensory function is normal, and thus check the normality of the anatomical system that sustains the function, without specific diagnosis; furthermore, EP provide quantitative functional measures and prognostic progression of the lesion. The two main data used in the interpretation of EP are the presence or absence of the P wave, as well as its morphological characteristics, and the latency of its appearance after the application of the stimulus.
Somatosensory evoked potentials are generated by stimulation of sensory peripheral nerves; the stimulated nerve fibers reach the posterior spinal ganglion, penetrate the spinal cord and cross over to the other side ascending within the medial lemniscus to reach the thalamus, from where impulses are relayed to the frontoparietal cortex, which records and evoke the answer to the initial stimulus. They are indicated in vascular lesions of the brainstem and cerebral hemispheres, mainly in thalamic alterations, as well as the study of comatose patients and brain death.
Visual evoked potentials are generated in response to nerve stimulation through variations in light intensity using a luminous board. Each eye is analyzed separately, and the evoked responses are collected in the occipital cortex. It is particularly suitable for the study of ischemic lesions of the optic nerve, and vascular alterations in the intracranial visual pathway.
Auditory evoked potentials are activated by applying acoustic stimuli separately for each ear, which are transmitted by the cochlear nerve, stimulating the auditory nuclei of the brainstem. In cerebrovascular pathology its primary indication is the study of comatose patients and brain death.
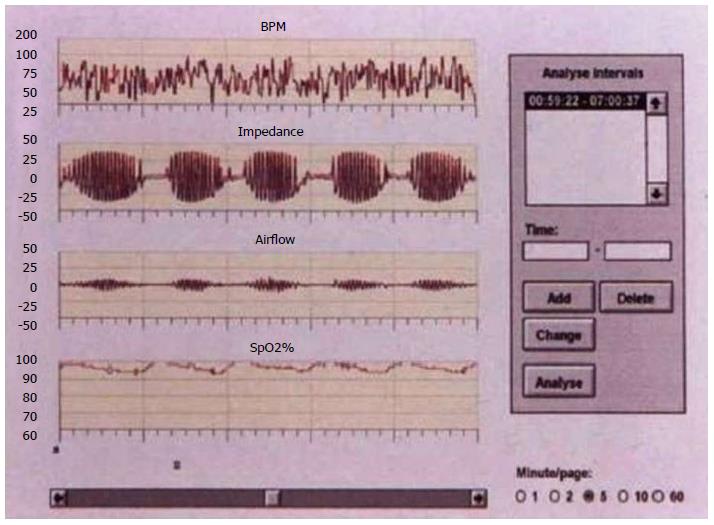
Obstructive sleep apnea syndrome (OSAS) is a new cardiovascular risk factor that results from intermittent and repetitive complete obstruction (apnea) or partial obstruction (hypopnea) of the upper airway during sleep, which manifests by sleep fragmentation and oxygen desaturation. The most common symptoms are daytime sleepiness, snoring and sleep apnea pauses.
A recent study conducted in 161 consecutive stroke patients showed that 71.4% of patients reported an apnea-hypopnea index > 10, which is compatible with OSAS, and 28% of them reported an index > 30 (severe OSAS) during the acute phase. Central respiratory disorders such as Cheyne-Stokes syndrome (Figure 1) may also occur.
Definitive diagnosis of respiratory sleep disorders associated with stroke has to be confirmed by overnight polysomnographic recording which simultaneously measures neurophysiological and cardiorespiratory variables. This technique allows for the assessment of the impact of apneas and hypopneas on cardiorespiratory function and sleep architecture. Polysomnography measures nasal/oral airflow, abdominal and thoracic wall movements (ventilator effort), transcutaneous oxygen saturation, electrocardiogram, and body position. Currently, in selected cases, continuous positive airway pressure (CPAP) during sleep is the treatment of choice for obstructive sleep apnea. Changes in cerebral hemodynamics can be detected in obstructive sleep apnea syndrome with greater neurological deterioration in the sitting of acute cerebral ischemia.
Approximately 55% of patients having a first lacunar infarct have mild cognitive impairment of the executive functions at the end of the acute phase of the disease and vascular dementia reaches 32% at 3 mo after the onset of symptoms[51].
In acute stroke, the initial vascular cognitive impairment is often the prelude to the subsequent vascular dementia and its subtypes: Multi-infarct or cortical predominant dementia, the strategic infarct dementia, Alzheimer’s disease with cerebrovascular disease and subcortical vascular dementia. Recurrent stroke is associated with an increased risk of vascular cognitive impairment, which in turn increases the risk of institutionalization and fatal outcome[52]. Antithrombotic drug therapy, statins in selected cases[53], control of stroke risk factors and non-drug therapy (physical exercise, healthy diet, avoid smoking and toxic habits) are essential for the secondary prevention of cerebral ischemia which is mandatory to prevent vascular dementia.
Recently it has been observed that asymptomatic carotid stenosis is also a risk factor for ischemic cognitive decline[54,55]. The pathophysiological mechanism whereby the carotid stenosis may cause some form of cognitive impairment can be multiple. It has been proven a decrease of approximately 25% of cerebral blood flow on the side of stenosis with respect to the contralateral side, and the long-lasting insufficient perfusion may impair energy metabolism in neurons and cause cognitive impairment[51]. In addition, cerebral magnetic resonance imaging based on weighted diffusion and gradient echo techniques suggest that either hemorrhagic and non-hemorrhagic microinfarcts or silent lacunes are far more common than clinically recognized. Beyond hypoperfusion and silent infarction, altered cerebrovascular reactivity and impaired regional functional connectivity have been associated with poorer cognitive performance in patients with asymptomatic carotid stenosis[54-56].
Conversely, neuropsychological impairments in subcortical lacunar infarcts probably result from the interruption of prefrontal-subcortical loops by lacunes and white matter lesions that result in executive dysfunction[57].
Some studies have reported conflicting results of carotid endarterectomy (CEA) and carotid artery stenting (CAS) on cognitive function. The mechanisms of CAS that can improve cognition are similar to those of CEA, including the improvement of cerebral perfusion and the reduction of the incidence of future brain infarctions[58].
The presence of brain atrophy can also play a major role in the cognitive decline associated with asymptomatic carotid disease[59]. This is an open line of research which will deserve further examination in the immediate future.
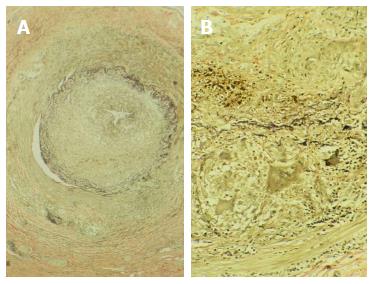
A temporal artery biopsy remains essential for the diagnosis of suspected giant cell arteritis or Horton’s disease (Figure 2). Stroke may cause the onset of this disease or be a serious complication in its clinical course. In a recent study, giant cell arteritis accounted for 5.7% of strokes of unusual cause admitted consecutively during a 10-year period. The pathological study of atherosclerotic plaques and arterial segments obtained by endarterectomy is also useful to contribute to the knowledge of the natural course of ulceration and thrombosis in atherogenesis. The digital artery biopsy may also be helpful in the diagnosis of Sneddon syndrome, since pathologic findings show focal skin ulceration and chronic inflammatory infiltrates with intimal thickening and occasionally thrombosis, without vasculitis.
Skin biopsies are useful to diagnose Fabry disease, secondary to alpha-galactosidase A deficiency that leads to glycosphingolipids accumulation in vascular endothelium and in other cells. Dermal lesions like diffuse corporal angiokeratoma are observed, coexisting with polyneuropathy, renal failure, heart disease and cerebrovascular disease. Skin biopsy shows characteristic dense inclusions with “fingerprint” patterns on endothelial cells, pericytes, fibroblasts and Schwann cells, both in clinically visible capillary lesions and in apparently healthy skin.
In CADASIL Syndrome (Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), an eosinophilic granular material deposit is observed in the leptomeningeal and perforating arterioles as well as thickening of basal lamina of the vascular smooth muscle by granular osmiophilic material seen as dense material by electron microscopy.
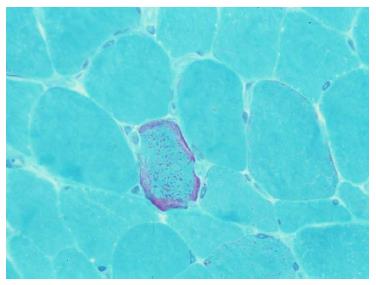
Muscle biopsy is an established test to diagnose mitochondrial encephalomyopathy, mainly in MELAS syndrome (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Muscle biopsy showing ragged red fibers visible under modified Gomori trichrome stain is consistent with mitochondrial myopathy. Cerebral ischemia might be related to true mitochondrial angiopathy located in the brain microcirculation (Figure 3).
Nerve biopsy is a useful procedure in patients with suspected systemic vasculitis or connectivopathy (mainly Wegener’s disease and polyarteritis nodosa).
Meningeal biopsies allow the visualization of the arterial vessels of the meningocortical network. The main indication is for patients with suspected isolated or granulomatous angiitis of the central nervous system. It may also be helpful in diagnosing intravascular lymphomatosis (former malignant angioendotheliosis of the central nervous system).
Intracerebral hemorrhages requiring surgical evacuation must be considered from a pathological point of view to rule out the presence of massive hemorrhage in primary or metastatic brain tumor, to detect cryptic vascular malformations causing bleeding and for the definitive diagnosis of cerebral amyloid angiopathy. Intravascular lymphomatosis and angiocentric T-cell lymphoma are two very unusual entities that involve neoplastic proliferation of lymphocytes and whose diagnosis is usually made by brain biopsy.
The authors thank Drs. E Comes and A Paipa for their insightful comments. This paper is dedicated to the memory of our wonderful colleague Professor Josep Lluis Martí Vilalta who recently passed away.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Altamura C, Radenovic L S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Arboix A. Diagnostic methods in cerebral vascular diseases. 2nd ed. Ergon: Madrid 2006; . |
| 2. | Arboix A. Diseases simulating transitory ischemic attacks or established strokes. Neurologia. 2002;17:353-354. [PubMed] |
| 3. | Libman RB, Wirkowski E, Alvir J, Rao TH. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122. [PubMed] |
| 4. | Martí-Vilalta JL, Arboix A, Vázquez J. Additional tests in cerebral vascular diseases. Neurologia. 1993;8:295-307. [PubMed] |
| 5. | Kalita J, Singh RK, Misra UK. Cerebral Salt Wasting Is the Most Common Cause of Hyponatremia in Stroke. J Stroke Cerebrovasc Dis. 2017;26:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Arboix A, Massons J, García-Eroles L, Oliveres M, Targa C. Diabetes is an independent risk factor for in-hospital mortality from acute spontaneous intracerebral hemorrhage. Diabetes Care. 2000;23:1527-1532. [PubMed] |
| 7. | Castellanos M, Castillo J, Dávalos A. Laboratory studies in the investigation of stroke. Handb Clin Neurol. 2009;94:1081-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Shanmugam V, Zimnowodzki S, Curtin J, Gorelick PB. Hypoglycemic hemiplegia: insulinoma masquerading as stroke. J Stroke Cerebrovasc Dis. 1997;6:368-369. [PubMed] |
| 9. | Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303-1306. [PubMed] |
| 10. | MacWalter RS, Wong SY, Wong KY, Stewart G, Fraser CG, Fraser HW, Ersoy Y, Ogston SA, Chen R. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. Stroke. 2002;33:1630-1635. [PubMed] |
| 11. | Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The Cardiovascular Health Study. Stroke. 1996;27:1479-1486. [PubMed] |
| 12. | Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28:557-563. [PubMed] |
| 13. | Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, Gillman MW, Kemper AR, Krist AH, Kurth AE. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1997-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 440] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 14. | [Cerebrovascular Diseases]; Catalan Society of Neurology. Official Guidelines for diagnosis and treatment. 2nd ed. Barcelona: Societat Catalana de Neurologia 2011; 159-240. |
| 15. | Adams HP, Brott TG, Crowell RM, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Marler JR, Woolson RF, Zivin JA. Guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:1901-1914. [PubMed] |
| 16. | Dávalos A, Ricart W, Gonzalez-Huix F, Soler S, Marrugat J, Molins A, Suñer R, Genís D. Effect of malnutrition after acute stroke on clinical outcome. Stroke. 1996;27:1028-1032. [PubMed] |
| 17. | Arboix A, Besses C, Acín P, Massons JB, Florensa L, Oliveres M, Sans-Sabrafen J. Ischemic stroke as first manifestation of essential thrombocythemia. Report of six cases. Stroke. 1995;26:1463-1466. [PubMed] |
| 18. | Arboix A, Martí-Vilalta JL. New concepts in lacunar stroke etiology: the constellation of small-vessel arterial disease. Cerebrovasc Dis. 2004;17 Suppl 1:58-62. [PubMed] |
| 19. | Chamorro A, Obach V, Cervera A, Revilla M, Deulofeu R, Aponte JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. 2002;33:1048-1052. [PubMed] |
| 20. | Caplan LR. Caplans’s Stroke: a clinical approach. Boston: Butterworth 2000; . |
| 21. | Kikta DG, Devereaux MW, Chandar K. Intracranial hemorrhages due to phenylpropanolamine. Stroke. 1985;16:510-512. [PubMed] |
| 22. | Martí-Vilalta JL. Enfermedades vasculares cerebrales. 2nd ed. Barcelona: Prous Science 2004; . |
| 23. | Ois A, Roquer J. Estudio inmunológico. Métodos diagnósticos en las enfermedades vasculares cerebrales. 2nd ed. Madrid: Ergon 2006; 83-94. |
| 24. | Rebollo M, Val JF, Garijo F, Quintana F, Berciano J. Livedo reticularis and cerebrovascular lesions (Sneddon’s syndrome). Clinical, radiological and pathological features in eight cases. Brain. 1983;106:965-979. [PubMed] |
| 25. | Arboix A, Massons J, Oliveres M, Navarro C, Domínguez MC, Ortega A, Titus F. Cerebral infarct in a young adult, as the presenting form of myeloencephalopathic syndrome with lactic acidosis and cerebral ischemia. Med Clin (Barc). 1990;94:457-460. [PubMed] |
| 26. | Moosy J, Bauer RB, Fletcher AP, García JH, Geer JC, McCormick WF. Report of the Joint Committee for Stroke Facilities. III. The laboratory evaluation of neurovascular disease (Stroke). Stroke. 1972;3:505-526. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 27. | Montaner J, Perea-Gainza M, Delgado P, Ribó M, Chacón P, Rosell A, Quintana M, Palacios ME, Molina CA, Alvarez-Sabín J. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 226] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 28. | Arboix A, Padilla I, Massons J, García-Eroles L, Comes E, Targa C. Clinical study of 222 patients with pure motor stroke. J Neurol Neurosurg Psychiatry. 2001;71:239-242. [PubMed] |
| 29. | Martí-Vilalta JL, Arboix A. The Barcelona Stroke Registry. Eur Neurol. 1999;41:135-142. [PubMed] |
| 30. | Martínez-Vila E, Noé E. Exploraciones complementarias generales en el paciente con ictus. Castillo J, Álvarez-Sabin J, Martí-Vilalta JL, Martínez-Vila E, Matías-Guiu J, editors. 2nd ed. Barcelona: Prous Science 1999; 243-252. |
| 31. | Salgado AV, Furlan AJ, Keys TF, Nichols TR, Beck GJ. Neurologic complications of endocarditis: a 12-year experience. Neurology. 1989;39:173-178. [PubMed] |
| 32. | Arboix A, Bechich S, Oliveres M, García-Eroles L, Massons J, Targa C. Ischemic stroke of unusual cause: clinical features, etiology and outcome. Eur J Neurol. 2001;8:133-139. [PubMed] |
| 33. | Kaste M, Roine RO. General stroke management and stroke units. Stroke. Pathophysiology, diagnosis and management. 5thed. Philadelphia: Elsevier 2011; 992-1007. |
| 34. | Kelley RE, Bell L, Kelley SE, Lee SC. Syphilis detection in cerebrovascular disease. Stroke. 1989;20:230-234. [PubMed] |
| 35. | Obach V. Estudio genético. Métodos diagnósticos en las enfermedades vasculares cerebrales. 2nd ed. Madrid: Ergon 2006; 325-342. |
| 36. | Rubenstein E, Federman DD, editors . Valores normales de laboratorio. México DF: Editora Científico Médica Latinoamericana 1987; . |
| 37. | Fishman RA. Cerebrospinal fluid in cerebrovascular disorders. Pathophysiology, diagnosis and management. Barnett HJM, Stein BM, Mohr JP, Yatsu FM, editors. New York: Churchill Livingstone 1986; 109-117. |
| 38. | Schluep M, Bogousslavsky J. Cerebrospinal fluid in cerebrovascular diseases. Ginsberg MD, Bogousslavsky J, editors. Malden: Blackwell Science 1998; 1221-1226. |
| 39. | Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637-676. [PubMed] |
| 40. | The diagnostic spinal tap. Health and Public Policy Committee, American College of Physicians. Ann Intern Med. 1986;104:880-886. [PubMed] |
| 41. | Lee SH, Rao KCVG. Cranial computed tomography and MRI. 2nd ed. New York: McGraw Hill 1987; . |
| 42. | Lee BC, Kneeland JB, Deck MD, Cahill PT. Posterior fossa lesions: magnetic resonance imaging. Radiology. 1984;153:137-143. [PubMed] |
| 43. | Marton KI, Gean AD. The spinal tap: a new look at an old test. Ann Intern Med. 1986;104:840-848. [PubMed] |
| 44. | Mauguière F. EEG and evoked potentials. Ginsberg MD, Bogousslavsky J, editors. Malden: Blackwell Science 1998; 1337-1345. |
| 45. | Chiappa KH, Ropper AH. Evoked potentials in clinical medicine (first of two parts). N Engl J Med. 1982;306:1140-1150. [PubMed] |
| 46. | Chiappa KH, Ropper AH. Evoked potentials in clinical medicine (second of two parts). N Engl J Med. 1982;306:1205-1211. [PubMed] |
| 47. | Parra O, Arboix A, Bechich S, García-Eroles L, Montserrat JM, López JA, Ballester E, Guerra JM, Sopeña JJ. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375-380. [PubMed] |
| 48. | Parra O, Sánchez-Armengol A, Bonnin M, Arboix A, Campos-Rodríguez F, Pérez-Ronchel J, Durán-Cantolla J, de la Torre G, González Marcos JR, de la Peña M. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Bonnin-Vilaplana M, Arboix A, Parra O, García-Eroles L, Montserrat JM, Massons J. Cheyne-stokes respiration in patients with first-ever lacunar stroke. Sleep Disord. 2012;2012:257890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Alexandrov AV, Nguyen HT, Rubiera M, Alexandrov AW, Zhao L, Heliopoulos I, Robinson A, Dewolfe J, Tsivgoulis G. Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke. 2009;40:2738-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Grau-Olivares M, Arboix A. Mild cognitive impairment in stroke patients with ischemic cerebral small-vessel disease: a forerunner of vascular dementia? Expert Rev Neurother. 2009;9:1201-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Arboix A, Font A, Garro C, García-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psychiatry. 2007;78:1392-1394. [PubMed] |
| 53. | Arboix A, García-Eroles L, Oliveres M, Targa C, Balcells M, Massons J. Pretreatment with statins improves early outcome in patients with first-ever ischaemic stroke: a pleiotropic effect of statins or a beneficial effect of hypercholesterolemia? BMC Neurol. 2010;10:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Buratti L, Balucani C, Viticchi G, Falsetti L, Altamura C, Avitabile E, Provinciali L, Vernieri F, Silvestrini M. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke. 2014;45:2072-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Lin CJ, Tu PC, Chern CM, Hsiao FJ, Chang FC, Cheng HL, Tang CW, Lee YC, Chen WT, Lee IH. Connectivity features for identifying cognitive impairment in presymptomatic carotid stenosis. PLoS One. 2014;9:e85441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Teng Z, Dong Y, Zhang D, An J, Lv P. Cerebral small vessel disease and post-stroke cognitive impairment. Int J Neurosci. 2016; Nov 28; Epub ahead of print. [PubMed] |
| 57. | Grau-Olivares M, Arboix A, Bartrés-Faz D, Junqué C. Neuropsychological abnormalities associated with lacunar infarction. J Neurol Sci. 2007;257:160-165. [PubMed] |
| 58. | Chen YH, Lin MS, Lee JK, Chao CL, Tang SC, Chao CC, Chiu MJ, Wu YW, Chen YF, Shih TF. Carotid stenting improves cognitive function in asymptomatic cerebral ischemia. Int J Cardiol. 2012;157:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Duering M, Righart R, Csanadi E, Jouvent E, Hervé D, Chabriat H, Dichgans M. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79:2025-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 60. | Caselli RJ, Hunder GG, Whisnant JP. Neurologic disease in biopsy-proven giant cell (temporal) arteritis. Neurology. 1988;38:352-359. [PubMed] |