Published online May 16, 2017. doi: 10.12998/wjcc.v5.i5.178
Peer-review started: January 12, 2017
First decision: February 17, 2017
Revised: February 20, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 16, 2017
Processing time: 126 Days and 13.9 Hours
Ticagrelor is a potent, direct P2Y12 antagonist with rapid onset of action and intense platelet inhibition, indicated in patients with acute coronary syndromes (ACS). This drug is usually well tolerated, but some patients experience serious adverse effects: Major bleeding; gastrointestinal disturbances; dyspnoea; ventricular pauses > 3 s. Given the unexpected high incidence of bradyarrhythmias, a PLATO substudy monitored this side effect, showing that ticagrelor was associated with an increase in the rate of sinus bradycardia and sinus arrest compared to clopidogrel. This side effect was usually transient, asymptomatic and not associated with higher incidence of severe atrioventricular (AV) block or pacemaker needs. A panel of experts from Food and Drug Administration did not consider bradyarrhythmias a serious problem in clinical practice and, accordingly, current labeling of the drug does not give any precaution or contraindication regarding this issue. However, recently some articles have described ACS patients with high-degree, life-threatening, AV block requiring drug discontinuation and, in some cases, pacemaker implantation. In this paper, we describe and discuss five published case reports of severe AV block following ticagrelor therapy and two other cases managed in our Hospital. The analysis of literature suggests that, although rarely, ticagrelor can be associated with life-threatening AV block. Caution and careful monitoring are required especially in patients with already compromised conduction system and/or treated with AV blocking agents. Future studies, with long-term rhythm monitoring, would help to define the outcome of patients at higher risk of developing this complication.
Core tip: Ticagrelor is a potent, direct antiplatelet agent with rapid onset of action and intense platelet inhibition, indicated in patients with acute coronary syndromes (ACS). Even if well tolerated, some patients experience bradyarrhythmias complications, especially sinus bradycardia and sinus arrest. This effect is usually transient, asymptomatic and not associated with higher incidence of severe atrioventricular block. However, recent articles have described ACS patients with high-degree atrioventricular block requiring drug discontinuation and, in some cases, pacemaker implantation. In this paper, we describe and discuss five published reports and two other cases managed in our Hospital. We conclude that, although rarely, ticagrelor can be associated with life-threatening atrioventricular block. Caution and careful monitoring are required especially in patients with already compromised conduction system and/or treated with atrioventricular blocking agents. Future studies, with long-term rhythm monitoring, would help to define the outcome of patients at higher risk of developing this complication.
- Citation: De Maria E, Borghi A, Modonesi L, Cappelli S. Ticagrelor therapy and atrioventricular block: Do we need to worry? World J Clin Cases 2017; 5(5): 178-182
- URL: https://www.wjgnet.com/2307-8960/full/v5/i5/178.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i5.178
Ticagrelor is a potent, direct P2Y12 antagonist with rapid onset of action and intense platelet inhibition. Unlike clopidogrel and prasugrel, it is not a thienopyridine and is not a prodrug. In patients with acute coronary syndromes (ACS) ticagrelor was superior to clopidogrel in reducing major adverse cardiac events and had a similar efficacy compared to prasugrel[1].
Ticagrelor is usually well tolerated, but some patients can experience serious adverse effects: Major bleeding (but rates are lower compared with other potent antiplatelet agents); gastrointestinal disturbances; dyspnoea; ventricular pauses > 3 s[1].
Given the unexpected high incidence of ventricular pauses in the landmark PLATO trial, this side effect was monitored by a prospectively designed, continuous electrocardiograph (ECG) monitoring substudy, including about 3000 patients[2]. In this study ticagrelor was associated with an increase in the rate of ventricular pauses > 3 s compared to clopidogrel (5.8% vs 3.6%, RR = 1.61, P = 0.01), mainly due to sinoatrial nodal pauses. This finding was only seen during the first week of therapy, while the incidence at 30 d was very low and similar between the two groups. Moreover, the great majority of pauses was asymptomatic and - even more important - there was no differences in the incidence of atrioventricular (AV) block or pacemaker need between groups[2].
As a consequence of this study[2] and after an “ad hoc” Food and Drug Administration meeting in 2011[3], a panel of experts concluded that the overall benefit of ticagrelor was superior to the risk of ventricular pauses, which appeared to be devoid of serious clinical consequences. Accordingly, current labeling of the drug does not give any precaution or contraindication regarding bradyarrhythmic effects.
Recently, 5 reports of ACS patients in a “real world clinical scenario” have been published, describing cases of severe bradyarrhythmias due to AV block requiring intensive care, temporary pacing and sometimes the implant of a permanent pacemaker.
The first article was published by Goldberg et al[4] in 2015. A 52-year-old diabetic man with ACS and severe stenosis of ostial left anterior descending (LAD) artery underwent 2 bare metal stents implantation. Baseline ECG showed complete right bundle branch block (RBBB). Left ventricular ejection fraction (LVEF) was preserved. The patient, already taking bisoprolol 1.25 mg, was also treated with a loading dose of ticagrelor 180 mg. A few hours later, several episodes of paroxysmal AV block occurred, with pauses > 11 s and syncope, requiring the insertion of a temporary pacing system. Subsequently, bisoprolol was stopped and ticagrelor replaced with clopidogrel. After 3 d, the AV block resolved and temporary pacing was removed without implanting a permanent pacemaker. At 6 mo follow up, no AV block or other bradyarrhythmias were recorded.
Ünlü et al[5] reported about a patient who developed symptomatic Mobitz type II AV block four days after receiving ticagrelor therapy in the context of ACS and left circumflex artery (LCA) stenting. The patient was already on beta-blocker therapy (bisoprolol 1.25 mg) before this acute event and baseline ECG showed first-degree AV block with narrow QRS. Ticagrelor and beta-blocker were withdrawn, but AV block still persisted after ten days, so a dual-chamber permanent pacemaker was implanted.
Goldberg et al[6] published the case of a 71-year-old female patient with ACS and proximal LAD occlusion, treated with thrombus aspiration and stent implantation. On ECG, she had complete left bundle branch block (LBBB) and was not taking beta-blockers. LVEF was moderately decreased. Ticagrelor was soon started, with recommended loading dose of 180 mg and continued with 90 mg twice a daily. Two days later, bisoprolol was started at 1.25 mg and after three hours complete AV block appeared, associated with sinus bradycardia, pauses up to 14 s and syncope. Temporary pacing was soon initiated, ticagrelor and bisoprolol were stopped. In two days AV block disappeared and temporary pacemaker was removed. A permanent pacemaker was not implanted and, at 6 mo follow up, no recurrence of AV block or other bradyarrhythmias were seen.
In the paper by Ozturk et al[7], a 62-year-old male diabetic patient (already on beta-blocker therapy) was admitted because of ACS and treated with right coronary artery (RCA) angioplasty. Baseline ECG showed first-degree AV block with narrow QRS. Seven hours after starting the 180 mg ticagrelor loading dose, a second-degree Mobitz II type AV block appeared, associated with sinus bradycardia. The bradyarrhythmia was asymptomatic and well tolerated. Beta-blocker was stopped but AV block persisted up to seven days, so ticagrelor was replaced with prasugrel. On the third day after ticagrelor withdrawal, AV block disappeared. The patient was discharged and after one month he did not experience any other bradycardia.
Lastly, Baker et al[8] described a 56-year-old male diabetic patient with ACS and severe proximal LAD stenosis, treated with drug-eluting stent (DES) implantation. At baseline ECG PR interval and QRS complex were normal. One hour after starting ticagrelor loading dose, PR interval increased to 204 ms, so beta-blocker was not started. After additional three hours, the patient experienced nausea, diaphoresis and lightheadedness, with telemetry strip showing severe sinus bradycardia, sinus arrests and paroxysms of AV block. An emergent coronary angiography revealed a widely patent LAD stent and a temporary pacing system was inserted. Ticagrelor was discontinued and replaced with prasugrel; after 12 h bradyarrhytmias completely resolved. After some days, low dose beta-blocker was introduced and subsequent clinical course was uneventful.
Here we describe two cases of ACS patients managed at our hospital, both with severe AV block following initiation of ticagrelor therapy.
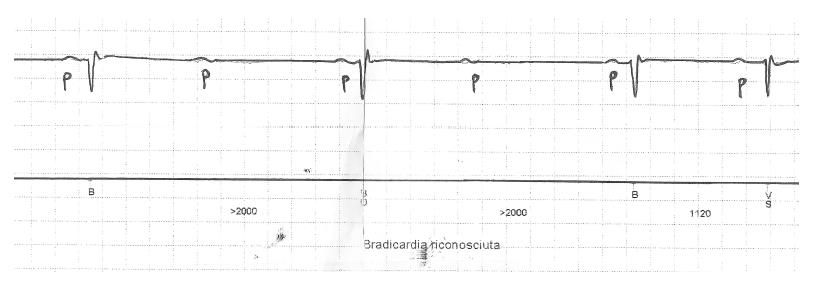
The first was an 82-year-old male patient admitted with ACS and severe proximal LAD stenosis, who was treated with DES implantation and ticagrelor. He was already taking bisoprolol 1.25 mg. At baseline ECG PR interval was prolonged (about 280 ms) and QRS complex was narrow. A few days after discharge, the patient was admitted again because of several syncopal episodes without prodromes. Continuous ECG monitoring showed several paroxysmal episodes of 2:1 AV block associated with lengthening of PP interval (associated sinus bradycardia); these episodes persisted even after bisoprolol discontinuation (Figure 1), but did not require temporary pacing. It was decided to replace ticagrelor with clopidogrel: After some days AV block resolved, without the need of a pacemaker, and bradycardia did not recur over 6 mo follow up.
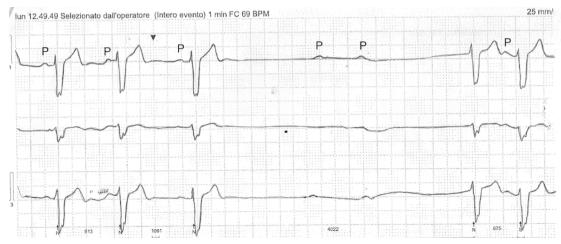
The second patient was a 76-year-old diabetic male with a recent DES implantation for LCA stenosis, in the setting of ACS hospitalization. Ticagrelor was started at usual doses just before angioplasty, while he was not taking beta-blocker because his baseline ECG displayed complete RBBB, left anterior hemiblock and a PR interval of 200 ms. Two weeks after starting ticagrelor, the patient was evaluated for recurrent syncopal episodes. A 24-h Holter ECG showed several episodes of paroxysmal complete AV block associated with PP interval lengthening (Figure 2). The patient was hospitalized and ticagrelor was replaced with prasugrel. During the following days, bradyarrhythmic phenomena were clearly reduced but did not completely disappear, so a permanent dual-chamber pacemaker was implanted.
The occurrence of ventricular pauses is a well-known side effect of ticagrelor, but it has been considered a transient phenomenon without serious clinical consequences. In this context, the most commonly reported arrhythmias are sinus bradycardia, sinus arrest and phases of junctional rhythm, usually fading away without symptoms. High-degree AV block occurred in a healthy volunteer after a large dose of the drug in a dose-finding study[1], but it was not considered a serious issue in the normal clinical setting[3]. It is only recently that some reports have described cases of high-degree, life-threatening, AV block requiring drug discontinuation[4-8], in patients with ACS.
The exact mechanisms of bradyarrhythmic effect of ticagrelor, leading to AV block, are not fully clear. It has been hypothesized a direct effect of the drug on cardiac automaticity and conduction, but the most plausible explanation is the increase in adenosine plasma concentration due to the inhibition of its cellular uptake[9]. Adenosine has a potent AV blocking effect and also a negative influence on the activity of the sinoatrial node[1,3,9]. Almost all the patients, in the above-described reports, displayed AV block associated with sinus node inhibition, manifesting as sinus bradycardia (PP interval lengthening during the block) or sinus arrest.
A total of seven ACS patients with severe bradyarrhythmia have been described in this paper (including our two cases) and six of them presented at baseline with an already diseased conduction system (first-degree AV block, LBBB, RBBB), which is a known risk factor for developing high-degree AV block. The insertion of a temporary pacing system was necessary in three patients with severe clinical picture. A permanent pacemaker was implanted in two patients with persistent high-degree AV block (one with pre-existing long PR interval, the other with baseline RBBB + left anterior hemiblock). Moreover, five patients out seven were taking beta-blocker therapy, which obviously increases the risk of bradyarrhythmias.
It is interesting to note that four patients of this series suffered from diabetes and it has been reported that cardiac conduction abnormalities occur more frequently in diabetic patients[10], even subclinically. The patient described by Baker et al[8] had normal PR interval and QRS duration but he was a diabetic. It is unclear how many patients had pre-existing conduction system disease in PLATO trial, while diabetes was present in 25% of the population[8]. In the PLATO substudy investigating the incidence of bradyarrhytmias[2], the majority of patients with ventricular pauses were also taking beta-blocker therapy.
There are several reasons why ticagrelor can reasonably be considered the offending agent in this series of ACS patients: (1) high-degree AV block appeared briefly after the drug was started; (2) high-degree AV block disappeared (or improved) after its discontinuation; (3) not all patients were taking beta-blocker therapy and - when prescribed - doses were low; (4) AV block did not resolve after beta-blocker withdrawal; and (5) there was no other clear explanation for such an acute arrhythmic event and coronary lesions involved all major arteries.
These observations suggest that ticagrelor can have life-threatening, although rare, bradyarrhythmic effects in patients with ACS. Caution and careful monitoring are required especially in patients with already compromised conduction system and/or treated with AV blocking agents (even if these conditions are not currently considered as contraindications to ticagrelor therapy). Moreover, it remains to be established whether ticagrelor treated patients with more stable cardiovascular diseases (chronic stable coronary artery disease, peripheral artery disease)[11,12] or with cerebral ischemia[13] have a lower risk of bradyarrhythmias compared to ACS patients.
Future studies, with long-term rhythm monitoring, would help to define the outcome of patients at higher risk of developing this complication, including the potential association with diabetes and the risk of bradyarrhytmias in clinical settings other than acute coronary events.
Two patients with acute coronary syndrome were treated with ticagrelor and developed high-degree atrioventricular block; drug was discontinued but one patient required permanent pacing anyway.
Acute coronary syndrome and iatrogenic atrioventricular block.
Primary atrioventricular block.
Troponin elevation, all other blood exams were within normal limits.
Atrioventricular block at electrocardiograph.
Non-ST-elevation myocardial infarction.
Drug discontinuation, pacemaker implant.
Recent articles have described patients with acute coronary syndrome treated with ticagrelor who developed high-degree atrioventricular block requiring drug discontinuation and, in some cases, pacemaker implantation.
Acute coronary syndrome is a condition with myocardial ischemia due to acute coronary occlusion; high degree atrioventricular block is a life-threatening bradyarrhythmia due to impaired conduction of atrial impulses to the ventricles.
Ticagrelor can have life-threatening, although rare, bradyarrhythmic effects in patients with acute coronary syndrome. Caution and careful monitoring are required especially in patients with already compromised conduction system and/or treated with atrioventricular blocking agents.
Comprehension and explanation of the problem is sound and the case-report is interesting.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Coccheri S, Culic V, Nam GB, Ozdemir S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Teng R, Butler K. Safety, tolerability, pharmacokinetics and pharmacodynamics of high single-ascending doses of ticagrelor in healthy volunteers. Int J Clin Pharmacol Ther. 2013;51:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Scirica BM, Cannon CP, Emanuelsson H, Michelson EL, Harrington RA, Husted S, James S, Katus H, Pais P, Raev D. The incidence of bradyarrhythmias and clinical bradyarrhythmic events in patients with acute coronary syndromes treated with ticagrelor or clopidogrel in the PLATO (Platelet Inhibition and Patient Outcomes) trial: results of the continuous electrocardiographic assessment substudy. J Am Coll Cardiol. 2011;57:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 3. | Gaglia MA, Waksman R. Overview of the 2010 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting regarding ticagrelor. Circulation. 2011;123:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Goldberg A, Rosenfeld I, Nordkin I, Halabi M. Life-threatening complete atrioventricular block associated with ticagrelor therapy. Int J Cardiol. 2015;182:379-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ünlü M, Demirkol S, Yildirim AO, Balta Ş, Öztürk C, Iyisoy A. Atrioventricular block associated with ticagrelor therapy may require permanent pacemaker. Int J Cardiol. 2016;202:946-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Goldberg A, Rosenfeld I, Nordkin I, Halabi M. Ticagrelor therapy in patients with advanced conduction disease: Is it really safe? Int J Cardiol. 2016;202:948-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Ozturk C, Unlu M, Yildirim AO, Erdogan S, Demir M, Balta S, Demirkol S, Celik T, Iyisoy A. The progressed atrioventricular block associated with ticagrelor therapy may not require permanent pacemaker after acute coronary syndrome; it may be reversible. Int J Cardiol. 2016;203:822-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Baker NC, Nadour W, Friehling M. Clinically significant ticagrelor induced conduction abnormalities following percutaneous coronary intervention. Int J Cardiol. 2016;214:21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 10. | Movahed MR. Diabetes as a risk factor for cardiac conduction defects: a review. Diabetes Obes Metab. 2007;9:276-281. [PubMed] |
| 11. | Ariotti S, Gargiulo G, Valgimigli M. Long-Term Use of Ticagrelor in Patients with Coronary Artery Disease. Curr Cardiol Rep. 2017;19:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Hiatt WR, Fowkes FG, Heizer G, Berger JS, Baumgartner I, Held P, Katona BG, Mahaffey KW, Norgren L, Jones WS. Ticagrelor versus Clopidogrel in Symptomatic Peripheral Artery Disease. N Engl J Med. 2017;376:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 13. | Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Jonasson J, Minematsu K, Molina CA. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med. 2016;375:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |