Published online Apr 16, 2017. doi: 10.12998/wjcc.v5.i4.140
Peer-review started: September 2, 2016
First decision: October 20, 2016
Revised: December 1, 2016
Accepted: January 2, 2017
Article in press: January 3, 2017
Published online: April 16, 2017
Processing time: 223 Days and 20.2 Hours
To evaluate the safety and efficacy of intragastric balloon (IGB) in weight reduction in obese patients referred to a tertiary hospital in the Kingdom of Saudi Arabia.
Three hundred and one consecutive obese individuals, who underwent IGB placement during January 2009 to May 2015, were analyzed. The subjects aged 18 to 60 years and had a minimum body mass index (BMI) of 27 kg/m2. The IGB was placed under conscious sedation and kept for 6 mo. Anthropometric measurements were recorded during and after 6 mo of IGB removal.
The body weight, excess body weight, and BMI were significantly reduced at the time of IGB removal and 6 mo later. Body weight loss > 10% was achieved in 224 subjects at removal of IGB. End of treatment success and long-term success were both significantly observed in women (70 vs 11) (71 vs 12.5) respectively. Excess BMI loss was significantly higher in subjects retaining the IGB for over 6 mo both at the removal [43.44 ± 19.46 (n = 221) vs 55.60 ± 28.69 (n = 80); t = 4.19, P = 0.0001] as well as at the end of 6 mo’ follow-up [46.57 ± 24.89 (n = 221) vs 63.52 ± 31.08 (n = 80); t = 4.87, P = 0.0001]. Within 3 d of IGB placement, two subjects developed pancreatitis and one subject developed cardiac arrhythmia. Intestinal obstruction due to displacement of IGB occurred in two subjects. All these subjects recovered uneventfully after immediate removal of the IGB.
IGB was effective in our cohorts. The observed weight reduction was maintained for at least 6 mo post IGB removal. IGB placement was safe with a satisfactory tolerance rate.
Core tip: Intragastric balloon (IGB) is a minimally invasive option for weight reduction. Several studies have demonstrated its superiority to lifestyle changes in reducing the morbidity and mortality associated with morbid obesity. This study evaluated the safety and efficacy of Medsil IGB in weight reduction of patients referred for weight reduction to a tertiary center in the Kingdom of Saudi Arabia. Endoscopic placement and keeping the Medsil IGB in situ for six months was proven to be safe, well tolerated and very effective for short and long term weight loss.
- Citation: Almeghaiseeb ES, Ashraf MF, Alamro RA, Almasoud AO, Alrobayan AA. Efficacy of intragastric balloon on weight reduction: Saudi perspective. World J Clin Cases 2017; 5(4): 140-147
- URL: https://www.wjgnet.com/2307-8960/full/v5/i4/140.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i4.140
Obesity, a medical condition in which body fat is accumulated in excess leading to severe negative health effects including reduced lifespan, affects an estimated 700 million people worldwide[1]. This pandemic health problem is much more serious threat to public health even when compared to alcohol consumption or tobacco smoking. Obesity decreases life expectancy by 6 to 7 years[2], while a body mass index (BMI) of 30-35 kg/m2 and > 40 kg/m2 reduces life expectancy by 2 to 4 years and by 10 years respectively[3]. Some estimates have even predicted that a BMI of > 40 reduces life expectancy by almost 20 years[4]. In addition to decline in the lifespan, this preventable cause of death also leads to quality of life deterioration owing to severe cardiologic, respiratory, dermatological, gastrointestinal, urinary, reproductive and psychiatric complications[5]. A recent estimate published in 2013 has put the overall prevalence of obesity at 28.7% (body mass index ≥ 30 kg/m2) in the Kingdom of Saudi Arabia, with women much more prone than men (33.5% vs 24.1%)[6].
Advanced cases of obesity require surgical interventions with drastic lifestyle modifications. Alternatively, mild to moderately obese subjects can achieve 5%-10% weight loss through exercise and dietary changes[7]. However, the weight gain recurs at high rates on cessation of these weight loss programs[8]. Further, pharmacological agents have not been found to be any better than dietary and exercise programs. The therapeutic or lifestyle management of obesity is a long-term and arduous undertaking. Literature shows that long-term treatments, both dietary regimens and weight-loss programs following pharmacotherapy remain largely ineffective[9]. Further, conservative treatment is clearly ineffective in morbid obesity (BMI ≥ 40 kg/m2)[10], while bariatric surgery remains the only option with promising long-term results. However, subjects who are unwilling to consent for or do not qualify for the bariatric surgery end up having intragastric balloon as the best possible alternative[11].
These factors have fostered a spurt in interest in the utility of intragastric balloon (IGB) to achieve weight loss in excess of 10%. While earlier studies have documented the utility of various intragastric balloons in Saudi subjects[12,13], data on newer variants of balloons is lacking. Hence, this study is an attempt to evaluate the end of treatment success rates (ETS) and long-term treatment success rates (LTS) for recently introduced, GOST R certified intragastric balloon MEDSIL® in obese patients referred to a tertiary health clinic in the Kingdom of Saudi Arabia.
Three hundred and one subjects, consecutively opting for IGB therapy for weight loss at The Prince Sultan Military Medical City Hospital, Kingdom of Saudi Arabia between January 2009 and May 2015 were included in the study. Both men and women aged 14 to 65 years with a minimum BMI of 27 kg/m2 and medically free from or with one or two of the comorbidities namely diabetes mellitus, hypertension, bronchial asthma, back pain, or Knee joint complains were included. In general, patients rated only up to ASA class II were preferred. Further, subjects without active endocrine diseases and ability to tolerate the procedure were selected for the procedure. Patients classified into ≥ ASA category were excluded from the procedure. Baseline characteristics of the study subjects are presented in Table 1.
| Characteristics | Average (mean ± SD)1 | Range |
| Age (yr) | 34.34 ± 10.38 | 14-65 |
| Female (%) | 67 | |
| Body weight (kg) | 94.73 ± 16.38 | 67-203 |
| Height (cm) | 161.52 ± 6.11 | 150-179 |
| BMI (kg/m2) | 36.24 ± 5.24 | 27.10-70.24 |
| Excess body weight (ideal BMI 25) | 29.42 ± 14.61 | 5.6-130.8 |
| Fasting blood glucose (mg/dL) | 100.15 ± 29.26 | 69-240 |
All the subjects underwent a routine clinical examination where information on anthropometrics and medical history was collected. Weight and height was measured with patients wearing no shoes and light clothing. Fasting blood sample was collected in the morning for the estimation of blood glucose concentration. Subsequent to the clinical evaluation of these results and after obtaining an informed written consent, MEDSIL® IGB was placed in the stomach through endoscopy.
All the subjects were treated with MEDSIL® IGB, silicon based saline filled bioenetric intragastric balloon (BIB) with a maximal volume of 700 mL (CSC MEDSIL, Russia). Patients were explained all the risks including perforation, bleeding, infection and adverse effects to the medicine, as well as the benefits and alternatives to the procedure prior to obtaining the informed written consent. All the patients understood the details and stated so.
For insertion of balloon, the patient was connected to monitoring devices and placed in left lateral position. In 297 patients, the device was implanted under procedural sedation and analgesia, with midazolam and fentanyl or pethidine. In 4 patients, the device implantation was done by inducing anesthesia using midazolam and intravenous propofol. Oxygen was provided continuously through a nasal cannula. Intravenous medications were administered through an indwelling cannula. After adequate conscious sedation was achieved, the patient was intubated and the endoscope was advanced under direct visualization to the duodenum.
Endoscope was withdrawn after complete examination for the presence of grossly anatomical contraindications. Balloon was inserted into the oral cavity and pushed into the stomach with a trocar followed by re-introduction of the endoscope. Under direct visualization, balloon was adjusted for proper placement followed by retraction of the push trocar wire. Balloon was then inflated with 400 mL to 700 mL of saline mixed with methylene blue. On achieving the desired inflation, the balloon catheter was gently pulled out leaving the balloon in stomach. The scope was gently retracted with careful examination of the colour, texture, anatomy, and integrity of the mucosa on the way out. The patient was subsequently transferred to the recovery area for observation.
The IGB was removed on completion of 6 mo, with the duration extending by one or more months in some subjects owing to various reasons. Anthropometric measurements were recorded during and after 6 mo of IGB removal. Overnight fasting blood specimen was collected at removal for the estimation of blood concentration. Patients were also provided with walk in follow-ups/clinic appointments on quarterly basis, but two third of the patients were seen on the fixed appointment only.
Similar protocol was followed for the removal of the balloon. After achieving adequate conscious sedation, the patient was intubated and the scope was advanced under direct visualization to the Stomach. After confirming absence of food or organic particles, an aspiration needle was inserted into the balloon followed by complete withdrawal of the fluid. The fully deflated balloon was withdrawn using a toothed forceps along the scope followed by routine follow up procedures.
Patients remained on their regular food without prescription of a hypocaloric diet. Post-insertion fasting blood glucose level estimation were scheduled and followed up to ensure that it was done as soon as treatment duration is completed.
Weight loss variables like body weight (kg), BMI, body weight loss (BWL%) and excess BMI loss (EBL%) were measured at baseline, and during and after 6 mo of IGB removal. A BWL value of > 10% at the time of IGB removal and after 6 mo of IGB removal was considered an ETS and LTS respectively. EBL% was calculated using the formula [(Baseline BMI-Current BMI)/(Baseline BMI-25)] × 100. All the descriptive data are expressed as mean ± SD. Paired t-test was used to compare baseline and outcome variables for individuals, whereas unpaired t-test was used for gender and age based comparisons. Fisher’s exact test was used to evaluate the occurrence of number of patients with BWL% > 10 between groups. The association of initial BMI and age with BWL% and EBL% was measured through Pearson correlation coefficient. A two-tailed P value of < 0.05 was considered statistically significant. Statistical Package for the Social Sciences (SPSS v. 18) was used for all the statistical tests.
Apart from the expected post procedure symptoms like nausea, vomiting and upper abdominal discomfort, and no serious complications were observed during recovery from IGB placement. Balloon was removed a day to week earlier than 6 mo in 20 subjects, at the completion of 6 mo in 201 subjects and after a week to few months over 6 mo in 80 subjects. In addition to the removal of IGB in 221 subjects owing to completion of the treatment duration, balloons were removed due to numerous other reasons as listed in the Table 2.
| Reason for removal | No. | Reason for removal | No. |
| TDC | 221 | Intolerance | 4 |
| Abdominal pain | 1 | Vomition | 1 |
| Miscellaneous | 2 | TDC and intolerance | 1 |
| TDC and abdominal pain | 19 | TDC and vomition | 10 |
| TDC and discomfort | 24 | TDC and other reasons | 2 |
| Intolerance and abdominal pain | 4 | Intolerance and vomition | 2 |
| Intolerance and other reason | 1 | Abdominal pain and vomition | 3 |
| Abdominal pain and other reason | 1 | Discomfort and other reason | 1 |
| TDC, abdominal pain and vomition | 1 | Intolerance, abdominal pain and vomition | 2 |
| TDC, abdominal pain and discomfort | 1 |
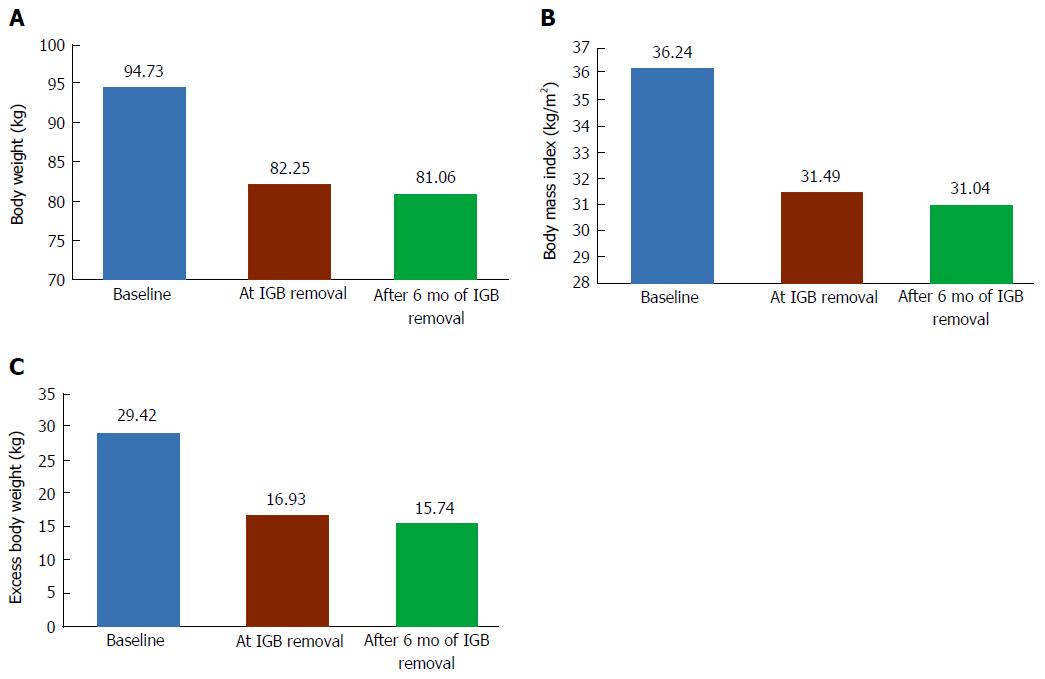
At the end of treatment, body weight, excess body weight, and BMI were significantly lowered as compared to initial measurements (Table 3 and Figure 1). The ETS rate, represented by number of patients with BWL% of > 10 was 74% (224 of 302 subjects). The fasting blood glucose remained statistically similar to the initial measurements. At the end of 6 mo after IGB removal, the body weight, excess body weight and BMI still remained significantly lower (Table 3). The BWL and BMI loss continued during post IGB removal phase, resulting in significantly higher measurements after 6 mo of removal as compared to the measurements taken during IGB removal. The LTS rates remained similar to ETS with just 2 more subjects added to the > 10% weight loss by the end of 6 mo IGB post-removal.
| Characteristics | At removal (mean ± SD)a | After 6 mo of removal |
| Body weight (kg) (Min-Max) | 82.25 ± 14.73b (55-181) | 81.06 ± 14.84b (53-181) |
| BWL (kg) (Min-Max) | 12.48 ± 5.16 (0-30) | 13.67 ± 6.65b (-1-42) |
| BWL (%) (Min-Max) | 13.08 ± 4.81 (0-35.29) | 14.30 ± 6.12b (-0.85-32.14) |
| No. of patients with BWL% > 10 | 224 | 226 |
| Excess body weight (ideal BMI 25) (Min-Max) | 16.93 ± 13.44b (-10.61-108.75) | 15.74 ± 13.73b (-12.72-107.04) |
| BMI (kg/m2) (Min-Max) | 31.49 ± 4.88b (20.96-62.63) | 31.04 ± 5.01b (20.44-63.14) |
| BMI loss (kg/m2) (Min-Max) | 4.75 ± 1.87 (0-11.43) | 5.20 ± 2.40b (-0.36-15.43) |
| EBMIL% (Min-Max) | 46.67 ± 22.88 (0-161.09) | 51.07 ± 27.66b (-2.12-195.53) |
| Fasting blood glucose (mg/dL) (Min-Max) | 98.67 ± 20.28 (71-187) | -- |
Statistical sub-analysis revealed different outcome when patients where compared according to gender and exercise habits (Table 4). The BWL%, BMI loss, and excess BMI loss (EBMIL%) was significantly lesser in women at the end of treatment as well as after 6 mo of removal. However, significantly higher proportion of women achieved ETS (70 vs 11) and LTS (71 vs 12.5) rates. As expected, BWL%, BMI loss and EBMIL% was significantly higher in exercising cohort at the end of treatment. Fasting blood glucose level changes remained statistically similar with gender and exercise habit.
| Characteristics | At removal (mean ± SD)a | After 6 mo of removal | ||||
| Gender | Exercise | Gender | ||||
| Male (n = 72) | Female (n = 229) | Yes (n = 131) | No (n = 170) | Male (n = 72) | Female (n = 229) | |
| BWL (%) | 14.99 ± 4.72 | 12.48 ± 4.68b | 15.22 ± 4.81 | 11.43 ± 4.11b | 16.71 ± 6.72 | 13.53 ± 5.72b |
| Number of patients with BWL% > 10 | 8 | 160b | 113 | 111b | 9 | 163b |
| Body mass index loss (kg/m2) | 5.56 ± 1.92 | 4.49 ± 1.78b | 5.47 ± 1.85 | 4.19 ± 1.68b | 6.19 ± 2.72 | 4.89 ± 2.20b |
| EBMIL% | 51.61 ± 24.75 | 45.12 ± 22.09a | 55.66 ± 25.12 | 39.74 ± 18.24b | 57.48 ± 30.27 | 49.06 ± 26.53a |
| Fasting blood glucose reduction (%) | -0.59 ± 13.29 | -0.92 ± 13.14 | -0.37 ± 13.10 | -1.25 ± 13.22 | -- | -- |
Age was not correlated with initial BMI or EMBIL% at initial or later phases of the study (Table 5). However, initial BMI was strongly correlated with BMI as well as EBMIL% measured at IGB removal as well as 6 mo after removal.
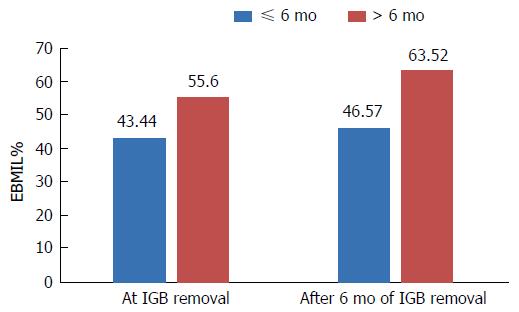
The duration of IGB removal was also important in determining the EBMIL% (Figure 2). The EBMIL% was significantly higher in subjects retaining the IGB for over 6 mo both at the removal [43.44 ± 19.46 (n = 221) vs 55.60 ± 28.69 (n = 80); t = 4.19, P = 0.0001] as well as at the end of 6 mo follow-up [46.57 ± 24.89 (n = 221) vs 63.52 ± 31.08 (n = 80); t = 4.87, P = 0.0001]. Duration of the IGB was also important in determining adverse complications in some of the individuals as outlined below.
In addition to the routine adverse events associated with IGB therapy, some of the patients experience unusual complication of spontaneous deflation and passage out of the digestive system. It must be noted that most of these patients had the balloon beyond the treatment duration. Out of five such cases, 2 women who had undergone IGB insertion at other institute, consulted us for removal after 1 year of insertion and three women operated at our clinic approached for removal at 8 mo. These five women underwent gastroscopy using X-ray and CT scan, which failed to reveal any traces of the IGB in the GI tract. The only plausible explanation for this is spontaneous rupture and excretion of the IGB without any knowledge of these patients.
One woman and one man developed clinical and biochemical pancreatitis on third day post IGB insertion. These symptoms subsided completely after immediate removal of IGB. One man developed arrhythmia 2 d post IGB insertion and recovered fully following immediate removal of IGB.
A woman with IGB got pregnant before the due date of removal and approached us at the end of first trimester with symptoms correlating intestinal obstruction. Esophagogastroduodenoscopy failed to detect balloon in the stomach. Imaging showed that balloon was lodged in middle of the jejunum. This IGB was surgically removed. The woman completed pregnancy without complications and gave birth to a normal offspring. Another man developed intestinal obstruction due to IGB dislodgement 7 mo post insertion that was detected through abdominal CT scan. This man recovered completely and uneventfully after laparoscopic surgery.
Subsequent to their introduction in 1982[14], numerous studies showed that IGBs are an effective and low cost method to achieve temporary weight loss in morbidly obese individuals, leading to significant decrease in morbidity and mortality rates[15,16]. The promising outcomes have fuelled the development of numerous fluid or air filled IGBs over the years. The newer variants are becoming much less invasive compared to surgical interventions in morbid obesity, though latter options remain primary approach in super-obese patients with a BMI over 50[17]. IGBs are also employed as a preoperative tool in bariatric surgery as its weight reducing effects significantly reduces the mortality, morbidity and risks associated with this invasive surgery[18].
Variety of intragastric balloons have been studied in numerous studies for safety and efficacy in Saudi subjects[12,19]. However, the newer variant of intragastric balloon Medsil remains to be tested in this population. In this study, we tested the end of the treatment and long term success rates for this device in a large Saudi cohort. Similar to a 2014 report on Czech subjects[20], these balloons were well tolerated in Saudi subjects analysed in this study.
This study demonstrated a clear benefit of Medsil balloon on body composition, as six mo placement of the balloon lead to significant reduction in body weight. The mean BMI loss (4.75 and 5.20 kg/m2) and BWL (13.08 and 14.30 kg) at IGB removal and after six months of removal were comparable to both the results of using other balloons or Medsil balloons. These studies have reported a BMI loss of 5.7-6.7 kg/m2 and weight loss of 14.7-17.8 kg[16,21,22], with one study using the same device reporting a BMI loss of 5.5 kg/m2 and weight loss of 18.4 kg[20]. Table 6 presents a comparison showing the similarities of outcomes in body composition reported by earlier studies. It must be noted that the BMI loss which corresponds to weight-loss was on the higher end as compared to other studies after one year of completion of the treatment.
| Study | Weight loss at removal (duration of placement in months) | Weight loss at follow-up (duration of follow-up in months) |
| Mathus-Vliegen et al[26], 2005 | 21.3 (12) | 12.6 (12) |
| Herve et al[27], 2005 | 12.0 (6) | 8.6 (12) |
| Doldi et al[28], 2004 | 15.5 (6) | -1.3 (14) |
| Melissas et al[29], 2006 | 41.6% EWL (6) | 23.9% EWL (6-30) |
| Angrisani et al[30], 2006 | 32.9% EWL (6) | 27.1% EWL |
| Ganesh et al[31], 2007 | 4.4 (6) | 1.5 (6-12) |
| Ohta et al[32], 2009 | 12 (6) | 6.4 (12) |
| Gümürdülü et al[33], 2013 | 12.4 (6) | 9.7 (6) |
| Bužga et al[20], 2014 | 18.4 (6) | - |
| Present study | 13.08 (6) | 14.30 (6) |
Two reviews have extensively evaluated the weight loss due to IGBs. Dumonceau[21] 2008 analysed 4877 patients from 30 studies and recorded a mean weight loss of 17.8 kg (or a BMI loss of 4-9 kg/m2). Another systematic review reported similar outcomes and revealed that, combined with lifestyle changes, IGBs provide an effective means for achieving a significant temporary weight loss, though the long term outcomes remain yet to be understood[16]. Our results of a significant weight loss and a large number of patients achieving and maintaining > 10% BWL from the IGB removal to follow-up after 6 mo, clearly suggests that long term results can be achieved through this method. In addition to the balloon, initial BMI, adherence to the lifestyle changes and patients level of motivation are highly likely to play an important role in achieving long term results.
Bioenetric intragastric balloons, which are now known as Orbera Intragastric Balloon (Apollo Endosurgery, Austin, TX, United States) are the most commonly used balloons. A comparison with the existing literature showed that BWL was less than expected, however not too less (Table 7).
| Balloon | Type (volume) | Material | Weight loss (EOT in months) | Ref. |
| Medsil BIB | Fluid-supplied (400-700 mL saline) | Silicone | 12.48 ± 5.16 kg (6 mo) | This study |
| Orbera (Apollo Endosurgery) | Fluid-supplied (400-700 mL saline) | Silicone | 16.9 ± 0.9 kg (6 mo) | Gaur et al[34], 2015 |
| The Elipse™ (Allurion Technologies) | Fluid-supplied (450-550 mL filling fluid) | NA | 2.4 kg (6 wk) | Machytka et al[35], 2016 |
| ReShape Duo® Integrated DualBalloon System (ReShape medical) | Fluid-supplied (900 mL; 450 mLX2 saline) | Silicone | 25.1 ± 1.6% EWL (6 mo) | Ponce et al[36], 2015 |
| Spatz Adjustable Balloon system (Spatz FGIA) | Fluid-supplied (400-600 mL saline) | Silicone | 24 kg (at 12 mo) | Brooks et al[37], 2014 |
| Heliosphere BAG® (Helioscopie) | Air-supplied (950 mL air) | Polyurethane and silicone | 16 ± 7 kg (6 mo) | Giardiello et al[38], 2012 |
| Obalon® Gastric Balloon (Obalon Therapeutics) | Air-supplied (250 mL air, nitrogen) | NA | 5 kg (12 wk) | Mion et al[39], 2013 |
The number of individuals achieving > 10% BWL increased from 224 to 226 from balloon removal to at 6 mo follow-up. This amounts to an increase in the number of patients achieving > 10% BWL during follow-up. These results are highly impressive as two of the earlier studies have showed that only 48%[23] or 55%[24] of the patients went on to continue losing weight from balloon removal to follow-up at 1 year. Our study results are very promising in this aspect.
The fasting blood glucose level remained statistically similar both during balloon removal as well as after 6 mo of follow-up. Bužga et al[20] 2014 also reported a similar result, though they demonstrated a positive effect of the balloon on glucose tolerance. On the contrary, earlier studies by Mathus-Vliegen and Konopko-Zubrycka have demonstrated a statistically significant reduction in fasting blood glucose levels through intragastric balloons[11,24]. These contradictory findings remain to be evaluated by meta-analysis to reveal the actual association.
Our study, though of higher strength due to large sample size, had a few limitations. A follow-up period of more than six months (at least 1 year) including tracking of comorbidities along with body conversion parameters would have been more insightful. The evaluation of fasting blood glucose levels could have been more meaningful if glucose tolerance and glycated hemoglobin levels were also included. Compared to earlier report of maintenance of > 10% weight loss in about 25% of patients for almost 30 mo[25], 75% of the subjects who achieved this result after 6 mo follow-up in our study seem to be responding much better. Looking at the similarities of BIB and Medsil balloons, it is highly likely that our subjects will be able to maintain weight loss for long term. However, it must be noted that our study provides the first report on the follow-up parameters for Medsil balloons.
In conclusion, it could be concluded that Medsil intragastric balloons are safe and effective for Saudi subjects and more than three fourth of the subjects can be expected to achieve long term weight loss.
Obesity is a major pan-endemic health problem in the Kingdom of Saudi Arabia affecting about 30% of the population. Literature shows that dietary regimens and weight-loss programs following pharmacotherapy remain largely ineffective. Bariatric surgery is the most effective long term option, however the majority are either reluctant to undergo surgery or do not qualify for medical reasons.
Intragastric balloons are of proven benefit as an alternative or a bridge to surgery, however the evidence for its utility particularly the newer version such as the intragastric balloon (IGB) MEDSIL® in the Kingdom of Saudi Arabia is lacking.
This study is an attempt to evaluate its long-term treatment success rate in obese patients referred to a tertiary health clinic in the Kingdom of Saudi Arabia. Endoscopic placement and keeping the Medsil IGB in situ for six months was proven to be safe, well tolerated and very effective for short and long term weight loss.
The intragastric balloons are well tolerated and are effective in weight reduction.
It is a retrospective study but of a very big cohort and the IGB is a new commercialized one. It is a well done paper and the language is good too.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Kingdom of Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de Castro ML, Doldi SB S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253. [PubMed] |
| 2. | Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 845] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 3. | Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083-1096. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3582] [Cited by in RCA: 3249] [Article Influence: 203.1] [Reference Citation Analysis (0)] |
| 4. | Ackroyd R, Mouiel J, Chevallier JM, Daoud F. Cost-effectiveness and budget impact of obesity surgery in patients with type-2 diabetes in three European countries. Obes Surg. 2006;16:1488-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Haslam DW, James WP. Obesity. Lancet. 2005;366:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3099] [Cited by in RCA: 3203] [Article Influence: 160.2] [Reference Citation Analysis (0)] |
| 6. | Memish ZA, El Bcheraoui C, Tuffaha M, Robinson M, Daoud F, Jaber S, Mikhitarian S, Al Saeedi M, AlMazroa MA, Mokdad AH. Obesity and associated factors--Kingdom of Saudi Arabia, 2013. Prev Chronic Dis. 2014;11:E174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 7. | Fisher BL, Schauer P. Medical and surgical options in the treatment of severe obesity. Am J Surg. 2002;184:9S-16S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Sheppard CE, Lester ELW, Whitlock KA, Karmali S, Birch DW, deGara CJ. Cost of Obesity Recurrence. Obesity and Diabetes, Springer, 2015; 11-33. . |
| 9. | Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1034] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 10. | Avenell A, Brown TJ, McGee MA, Campbell MK, Grant AM, Broom J, Jung RT, Smith WC. What are the long-term benefits of weight reducing diets in adults? A systematic review of randomized controlled trials. J Hum Nutr Diet. 2004;17:317-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Konopko-Zubrzycka M, Baniukiewicz A, Wróblewski E, Kowalska I, Zarzycki W, Górska M, Dabrowski A. The effect of intragastric balloon on plasma ghrelin, leptin, and adiponectin levels in patients with morbid obesity. J Clin Endocrinol Metab. 2009;94:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Subei IM, Abdelazim A, Bayoumi A, Wahab MA, El Deriny S. The effect of different types of intragastric balloons with and without a behavior modification program in morbid obesity. Saudi J Gastroenterol. 1996;2:63-68. [PubMed] |
| 13. | Tayyem RM, Obondo C, Ali A. Short-term outcome and quality of life of endoscopically placed gastric balloon and laparoscopic adjustable gastric band. Saudi J Gastroenterol. 2011;17:400-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Genco A, Cipriano M, Bacci V, Cuzzolaro M, Materia A, Raparelli L, Docimo C, Lorenzo M, Basso N. BioEnterics Intragastric Balloon (BIB): a short-term, double-blind, randomised, controlled, crossover study on weight reduction in morbidly obese patients. Int J Obes (Lond). 2006;30:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Imaz I, Martínez-Cervell C, García-Alvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg. 2008;18:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Milone L, Strong V, Gagner M. Laparoscopic sleeve gastrectomy is superior to endoscopic intragastric balloon as a first stage procedure for super-obese patients (BMI & gt; or =50). Obes Surg. 2005;15:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Genco A, Balducci S, Bacci V, Materia A, Cipriano M, Baglio G, Ribaudo MC, Maselli R, Lorenzo M, Basso N. Intragastric balloon or diet alone? A retrospective evaluation. Obes Surg. 2008;18:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Al Kahtani K, Khan MQ, Helmy A, Al Ashgar H, Rezeig M, Al Quaiz M, Kagevi I, Al Sofayan M, Al Fadda M. Bio-enteric intragastric balloon in obese patients: a retrospective analysis of King Faisal Specialist Hospital experience. Obes Surg. 2010;20:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Bužga M, Evžen M, Pavel K, Tomáš K, Vladislava Z, Pavel Z, Svagera Z. Effects of the intragastric balloon MedSil on weight loss, fat tissue, lipid metabolism, and hormones involved in energy balance. Obes Surg. 2014;24:909-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg. 2008;18:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Crea N, Pata G, Della Casa D, Minelli L, Maifredi G, Di Betta E, Mittempergher F. Improvement of metabolic syndrome following intragastric balloon: 1 year follow-up analysis. Obes Surg. 2009;19:1084-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Escudero Sanchis A, Catalan Serra I, Gonzalvo Sorribes J, Bixquert Jimenez M, Navarro Lopez L, Herrera Garcia L. Efectividad, seguridad y tolerancia del balón intragástricoasociado a unadieta hipocalórica para la reducción de peso en pacientesobesos. Rev Espaà Enferm Digest. 2008;100:349-354. |
| 24. | Mathus-Vliegen EM, Eichenberger RI. Fasting and meal-suppressed ghrelin levels before and after intragastric balloons and balloon-induced weight loss. Obes Surg. 2014;24:85-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Dastis NS, François E, Deviere J, Hittelet A, Ilah Mehdi A, Barea M, Dumonceau JM. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19-27. [PubMed] |
| 27. | Herve J, Wahlen CH, Schaeken A, Dallemagne B, Dewandre JM, Markiewicz S, Monami B, Weerts J, Jehaes C. What becomes of patients one year after the intragastric balloon has been removed? Obes Surg. 2005;15:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Doldi SB, Micheletto G, Perrini MN, Rapetti R. Intragastric balloon: another option for treatment of obesity and morbid obesity. Hepatogastroenterology. 2004;51:294-297. [PubMed] |
| 29. | Melissas J, Mouzas J, Filis D, Daskalakis M, Matrella E, Papadakis JA, Sevrisarianos N, Charalambides D. The intragastric balloon - smoothing the path to bariatric surgery. Obes Surg. 2006;16:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Angrisani L, Lorenzo M, Borrelli V, Giuffré M, Fonderico C, Capece G. Is bariatric surgery necessary after intragastric balloon treatment? Obes Surg. 2006;16:1135-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Ganesh R, Rao AD, Baladas HG, Leese T. The Bioenteric Intragastric Balloon (BIB) as a treatment for obesity: poor results in Asian patients. Singapore Med J. 2007;48:227-231. [PubMed] |
| 32. | Ohta M, Kitano S, Kai S, Shiromizu A, Eguchi H, Endo Y, Masaki T, Kakuma T, Yoshimatsu H. Initial Japanese experience with intragastric balloon placement. Obes Surg. 2009;19:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Gümürdülü Y, Doğan ÜB, Akın MS, Taşdoğan BE, Yalakı S. Long-term effectiveness of BioEnterics intragastric balloon in obese patients. Turk J Gastroenterol. 2013;24:387-391. [PubMed] |
| 34. | Gaur S, Levy S, Mathus-Vliegen L, Chuttani R. Balancing risk and reward: a critical review of the intragastric balloon for weight loss. Gastrointest Endosc. 2015;81:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Machytka E, Chuttani R, Bojkova M, Kupka T, Buzga M, Stecco K, Levy S, Gaur S. Elipse™, a Procedureless Gastric Balloon for Weight Loss: a Proof-of-Concept Pilot Study. Obes Surg. 2016;26:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 37. | Brooks J, Srivastava ED, Mathus-Vliegen EM. One-year adjustable intragastric balloons: results in 73 consecutive patients in the U.K. Obes Surg. 2014;24:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Giardiello C, Borrelli A, Silvestri E, Antognozzi V, Iodice G, Lorenzo M. Air-filled vs water-filled intragastric balloon: a prospective randomized study. Obes Surg. 2012;22:1916-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Mion F, Ibrahim M, Marjoux S, Ponchon T, Dugardeyn S, Roman S, Deviere J. Swallowable Obalon® gastric balloons as an aid for weight loss: a pilot feasibility study. Obes Surg. 2013;23:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |