Published online Mar 16, 2017. doi: 10.12998/wjcc.v5.i3.93
Peer-review started: September 6, 2016
First decision: September 29, 2016
Revised: October 10, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: March 16, 2017
Processing time: 192 Days and 16.6 Hours
Bayés syndrome is an under-recognized clinical condition characterized by advanced interatrial block. Bayés syndrome is a subclinical disease that manifests electrocardiographically as a prolonged P wave duration > 120 ms with biphasic morphology ± in the inferior leads. The clinical relevance of Bayés syndrome lies in the fact that is a clear arrhythmological syndrome and has a strong association with supraventricular arrhythmias, particularly atypical atrial flutter and atrial fibrillation. Likewise, Bayés syndrome has been recently identified as a novel risk factor for non-lacunar cardioembolic ischemic stroke and vascular dementia. Advanced interatrial block can be a risk for embolic stroke due to its known sequelae of left atrial dilation, left atrial electromechanical dysfunction or atrial tachyarrhythmia (paroxysmal or persistent atrial fibrillation), conditions predisposing to thromboembolism. Bayés syndrome may be responsible for some of the unexplained ischemic strokes and shall be considered and investigated as a possible cause for cryptogenetic stroke. In summary, Bayés syndrome is a poorly recognized cardiac rhythm disorder with important cardiologic and neurologic implications.
Core tip: Bayés syndrome is an under-recognized cardiac rhythm disorder with significant cardiologic and neurologic implications. It constitutes a genuine arrhythmological syndrome characterized by advanced interatrial block. Bayés syndrome is a key predictor of higher risk of new-onset atrial fibrillation and it is independently associated with an increased risk for non-lacunar cardioembolic stroke. Likewise, can be the cause of some cryptogenic strokes, and be related to clinically silent cerebral ischemia and vascular cognitive impairment, or even, vascular dementia.
- Citation: Arboix A, Martí L, Dorison S, Sánchez MJ. Bayés syndrome and acute cardioembolic ischemic stroke. World J Clin Cases 2017; 5(3): 93-101
- URL: https://www.wjgnet.com/2307-8960/full/v5/i3/93.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i3.93
Bayés syndrome is an under-recognized cardiological condition characterized by advanced interatrial block. Although it has yet to receive adequate coverage in textbooks and remains poorly perceived in clinical practice, Bayés syndrome represents a novel risk factor for cardioembolic ischemic stroke[1,2].
The principal goal of this mini-review is to expand and update knowledge of the little-known relationship between Bayés syndrome and acute ischemic cardioembolic stroke.
It should be noted that cardioembolic ischemic stroke accounts for one-quarter of all cerebral infarcts[3], is the most severe ischemic stroke subtype with a low prevalence of absence of neurological dysfunction at hospital discharge and a non-negligible risk of early embolic recurrence (1%-10%)[4-7], and has the highest in-hospital mortality (6%-27%)[3,4,8].
Compared to non-cardioembolic stroke, the percentage of female sex (54.3% vs 34.6%) and very old patients (≥ 85 years) (28.5% vs 18.3%) is more frequent. This may be explained by the increasing prevalence of atrial fibrillation with age. In the Framingham study, a growing population attributable risk of stroke due to atrial fibrillation with age was found, with a prevalence of atrial fibrillation of 1.8% in patients aged 60-69 years, 4.8% in those aged 70-79 years, and 8.8% in the 80 to 90 year group[9]. Similarly, the increased frequency of cardioembolic infarcts in women compared to non cardioembolic, which are more frequent in men, may also be related to increasing age observed in the industrialized societies, where women represent the majority of elderly people due to their higher life expectancy[10].
In the Sagrat Cor Hospital of Barcelona Stroke Registry (Table 1), which is one of the first stroke data banks of Catalonia and Spain, the short prognosis of patients with cardioembolic cerebral infarction is poorer compared to other subtypes of cerebral infarction with higher in-hospital mortality (21.9% vs 8.2%), whereas symptom free at discharge are less frequent (14.3% vs 19.9%)[7].
| Variable | Cardioembolic stroke n = 575 | Non-cardioembolic cerebral infarct1 n = 1507 | P value |
| Age, yr, mean (SD) | 78.96 (9.39) | 73.45 (12.8) | 0.0001 |
| Age strata, yr | 0.0001 | ||
| < 65 | 44 (7.6) | 285 (18.9) | |
| 65-74 | 116 (20.2) | 405 (26.9) | |
| 75-84 | 251 (43.7) | 557 (37.0) | |
| ≥ 85 | 164 (28.5) | 260 (17.3) | |
| Sex | 0.0001 | ||
| Males | 199 (34.6) | 788 (52.3) | |
| Females | 373 (65.4) | 719 (47.7) | |
| Hypertension | 291 (50.6) | 835 (55.4) | 0.049 |
| Diabetes | 103 (17.9) | 368 (24.4) | 0.002 |
| Atrial fibrillation | 433 (75.3) | 176 (11.7) | 0.0001 |
| Heavy smoking (> 20 cigarettes/d) | 23 (4.0) | 184 (12.2) | 0.0001 |
| ACM vascular topography | 391 (68.0) | 703 (46.6) | 0.0001 |
| Echocardiography | 363 (63.1) | 598 (39.7) | 0.0001 |
| Symptom-free at discharge | 82 (14.3) | 300 (19.9) | 0.003 |
| In-hospital death | 126 (21.9) | 123 (8.2) | 0.0001 |
| Transfer to convalescent/rehabilitation units | 89 (15.5) | 154 (10.2) | 0.001 |
| Length of stay, days, median (interquartile range) | 15 (10-24) | 11 (8-19) | 0.0001 |
| Prolonged hospital stay > 12 d | 330 (57.4) | 650 (43.1) | 0.0001 |
Recent studies have shown that Bayés syndrome is a key independent factor of cardioembolic cerebral ischemia[1,2], although there is still a need of high level of clinical suspicion in order to diagnose it. Early and proper diagnosis of Bayés syndrome is desirable and necessary, since patients will require closer clinical surveillance, and possibly accompanying antiarrhythmic and antithrombotic preventive therapies.
In analogy to other cardiac conduction delays, atrial conduction abnormalities should be divided into partial and advanced interatrial blocks (aIAB) or Bayés syndrome. The syndrome of advanced interatrial conduction block due to conduction impairment in Bachmann’s bundle, results in delayed and retrograde activation of the left atrium that signifies a conduction delay between the left and right atria, and it is associated with a high incidence of atrial tachyarrhythmias, especially a particular and specific form of atypical atrial flutter or atrial fibrillation[11,12].
The first case of inter-atrial block was described by Bachmann[13] in 1941. Later, in 1971, Castillo and Vernant[14] emphasized that when a P wave with plus/min (biphasic) morphology is observed in leads II, III, and avF, the atrial stimulus is blocked in the upper part of the septum. Finally, between 1979 and 1985, Bayes de Luna et al[15,16] precisely analyzed the prevalence, pathological associations, and profile of the arrhythmias associated with aIAB, thereby defining a distinct and well-defined anatomo-electrical entity. Dr. Bayés de Luna contribution was fundamental in demonstrating the association between advanced interatrial block and supraventricular arrhythmias, thus confirming a well-defined arrhythmic syndrome. The consensus of naming this association with the eponymous Bayés syndrome has recently been accepted by the scientific community in honor of Dr. Antoni Bayés de Luna, the great Catalan master of clinical electrocardiography[1,17,18], for his contribution to the understanding of the natural history of this cardiac syndrome. However, Bayés syndrome remains an under-recognized clinical condition.
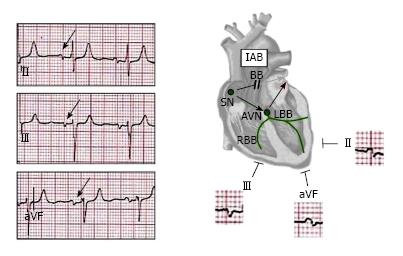
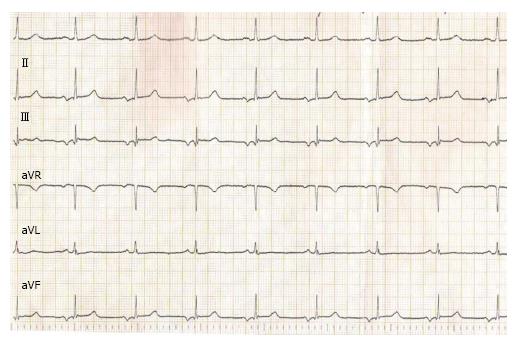
Bayés de Luna described the electrocardiographic pattern for identifying IAB and classified the types of block that occur at the atrial level. The distinction is based on the P-wave duration, and more important, the P-wave morphology: A partial block, indicated by a P-wave duration of 120 ms or more, and bifid P wave (notched P-wave) in leads II, III and aVF (Figure 1). If the interatrial block is advanced, also, the P wave is prolonged (duration 120 ms or more), but the second part of the P wave in inferior leads becomes negative (biphasic pattern or P-wave plus/min morphology) because of the retrograde activation of the left atrium (P-wave ± in II, III, and aVF) (Figure 2)[19-21].
It should be noted that, initially, IAB may occur transiently and may be reversible. It may be classified as first-degree (partial), second-degree (transient interatrial block or atrial aberrancy), or third-degree (advanced).There is consensus on considering transient interatrial block as a marker of electromechanical dysfunction of the left atrium and a risk factor for recurrence of atrial fibrillation[11,15].
Although the diagnosis of interatrial block is frequently associated with left atrial enlargement (LAE), there are some cases, especially of first-degree IAB, without this association. Therefore, it should be noted that IAB is a separate entity from atrial enlargement[11,22].
The prevalence of interatrial block is age-dependent, increasing from 5.4% at < 20 years old to 60% at > 50; in the same way, advanced IAB increases from 0.1% to 2% in patients with heart valve disease and cardiomyopathy[23,24]. The increased age-related risk may be probably due to atrial fibrosis which would result in impaired atrioventricular conduction through the atria. However, the exact pathogenesis has not been elucidated and various comorbidities, including coronary heart disease, arterial hypertension, and diabetes mellitus, have been proposed. The cause of IAB may be likely degenerative because of the increased incidence with age[11].
The Bayés syndrome is a clear arrhythmological syndrome. Advanced IAB is a key predictor for high risk of new-onset atrial fibrillation after a successful cavo-tricuspid isthmus ablation in patients with typical atrial flutter[11,25].
A clinical study reported that 90% of patients with atrial fibrillation recurrence at one year had advanced IAB, and multivariate analysis demonstrated that persistent IAB was a predictor of AF recurrence. Advanced IAB is a useful marker to identify subjects who are at high risk for developing atrial fibrillation, and is a pre-atrial fibrillation condition associated with premature atrial beats[24].
Practical consequences and clinical implications of Bayés syndrome are the high incidence of atrial extrasystoles and paroxysmal supraventricular tachyarrhythmia, especially in patients with valvular heart disease or cardiomyopathy. A control group of patients with similar clinical states and left atrial size by echocardiography showed much lower incidence of these arrhythmias[11]. Bayés de Luna et al[26] also suggested that antiarrhythmic treatment prevents recurrences of atrial tachyarrhythmia in these cases.
There are currently no evidence-based recommendations on the most appropriate therapeutic approach for Bayés syndrome in any of the different cardiologic or neurologic guidelines for primary or secondary prevention of cerebral ischemia. A clinical case of a patient with Bayés syndrome reported antiarrhythmic treatment with amiodarone and anticoagulant administration with acenocoumarol[27].
Prolonged QRS duration is an independent predictor of cardiovascular mortality in patients with underlying structural heart disease. Similarly, the relation between sudden death and QT prolongation is an established fact[11]. Increased P wave duration is the only P wave index significantly associated with increased cardiovascular mortality. Therefore, IAB as a subclinical disease merits elucidation as a marker of risk for adverse outcomes.
Recently, Bayés syndrome has been shown to be a predictor of cardioembolic stroke[28]. There are three main consequences of advanced IAB: Firstly, IAB is a substrate for sustained AF, and the association between AF and advanced IAB has been demonstrated. Secondly, IAB results in poor left atrium (LA) contractility due to a delayed depolarization which can result in LA dysfunction. Such a delay has hemodynamic consequences including raised LA pressure and LA dilatation, which again is a substrate for AF. Thirdly, IAB may be associated with structural factors as a result of left atrium enlargement, although it may occur in patients with normal left atrium size[11].
As a result, advanced IAB could be a risk for embolic stroke due to its known sequelae of left atrial dilation, LA electromechanical dysfunction or atrial tachyarrhythmias, conditions which predispose to the formation of echocontrast, and may serve as a nidus for thrombi or microthrombi, and thus increase the risk for cardioembolic events. Because IAB predicts atrial fibrillation, patients with IAB may intermittently be in atrial fibrillation (paroxysmal atrial fibrillation), causing embolization[3,11].
Ariyarajah et al[2] analyzed 293 patients with cerebral infarct, 85 of them cardioembolic, and reported that 88% of cardioembolic infarcts showed sinus rhythm and 61% of these had advanced IAB, concluding that IAB could be a novel risk factor for embolic stroke.
In an analysis of ARIC (Atherosclerosis risk in Communities Study) advanced IAB was independently associated with an increased risk for ischemic stroke, thus definitively confirming IAB as a novel risk factor for cardioembolic ischemic stroke[29].
Cotter et al[30] reported an increased incidence of interatrial block in younger adults with cryptogenic stroke and patent foramen ovale, suggesting atrial arrhythmias as a possible cause of unexplained ischemic stroke in these patients. In another study, atrial fibrillation detected by implantable loop recorders in unexplained stroke was identified in 25.5% of cases, and AF was independently associated with interatrial conduction block[31].
In a clinical study the CHADS2 and CHADS2DS2-VA SCc scores could predict the risk of ischemic stroke or TIA in patients with IAB without atrial fibrillation[32].
However, the association of Bayés syndrome and ischemic stroke is limited to non-lacunar cardioembolic infarcts[33,34]. Lacunar infarcts are an ischemic stroke subtype related mainly to hypertension and diabetes[35,36]. Ischemic stroke of unusual causes accounted for 5% of ischemic strokes and the association of advanced IAB in this ischemic stroke subtype is improbable[37].
By contrast, it is important to highlight that about 10%-30% of ischemic strokes remain cryptogenic despite reasonably thorough evaluations[38,39]. A possible explanation for this is that IAB may be responsible for some of the unexplained strokes.
Furthermore, atrial fibrillation is independently associated with an increased risk of vascular dementia. In a clinical study conducted in centenarians, the rate of dementia was 48% in subjects with a normal P wave, 60% in those with partial IAB, and 81% in those with advanced IAB and 90% in those with atrial fibrillation[40].
Table 2 shows the most relevant published studies about IAB as a cardiovascular risk factor and acute ischemic stroke[41-43].
| Ref. | Study type | n | Age (yr) | Gender | Inclusion criteria | Exclusion criteria | Confounding factors | Parameters evaluated | Results |
| Wu et al[32] | Retrospective cohort | 1046 | 63 ± 10 | 612 males 434 females | Patients hospitalized in Zhengzhou University People's Hospital for diagnosis and treatment between March 1 and March 31 of 2010 ECG Presence of IAB | History of AF Patients under anticoagulant treatment Missing data for calculation of CHADS2 and CHA2DS2-VASc scores Lost to follow-up | Congestive Heart Failure Hypertension Diabetes Mellitus Previous strokes/TIA Coronary Artery Disease PCI during index admission CABG during index admission Tobacco consumption LVEF LA diameter Medication Use | Conduction lengths CHADS2 and CHA2DS2-VASc scores Apparition of Stroke (Hemorrhagic or Ischemic) | Mean follow-up of 4.9 ± 0.7 yr 0.8% hemorrhagic stroke 5.3% presented ischemic stroke or TIA Ischemic stroke or TIA increased with CHADS2 score: 0.37, 0.85, 0.96 and 1.92 per 100-person years for scores of 0, 1, 2, and > 3 respectively CHA2DS2-VASc scores correlated with Ischemic stroke or TIA (0.19, 0.59, 0.76, 0.88, and 2.0 for scores of 0, 1, 2, 3, and > 4 respectively) Cut-off points: > 3 for CHADS2, > 4 for CHA2DS2-VASc Conclusion: CHADS2 and CHA2DS2-VASc scores may be predictors of risk of ischemic stroke or TIA in patients with IAB without atrial fibrillation |
| Martinez-Selles et al[40] | Case-control | 80 | 101.4 ± 1.5 | 21 males 59 females | Patients from the Cardiac and Clinical Characterization of Centenarians (4C) Registry | Hospitalized patients | Dementia Perceived health status score Previous stroke Mitral regurgitation Systolic dysfunction Left atrial diameter > 40 mm | Conduction lengths ECG measurements Short Portable Mental Status Questionnaire Premature atrial beats | IAB group showed higher rate of previous stroke than normal P wave and AF groups Premature atrial beats were more frequent in advanced IAB than normal P-wave Mitral regurgitation could play an important role in IAB Conclusion: Advanced IAB is a pre-atrial fibrillation condition associated with premature atrial beats. Atrial arrhythmias and IAB occurred more frequently in centenarians than in septuagenarians. |
| O'Neal et al[24] | Retrospective cohort | 14716 | 54 ± 5.8 | 6622 males 8094 females | Patients enrolled in the ARIC Study Recruited between 1987 and 1989 | Patients with prevalent stroke or AF at baseline Race other than black or white Black participants from Washington County and Minneapolis | Black Tobacco use Diabetes LDL cholesterol level BMI Hypertension Antihypertensive medication Coronary heart disease Heart failure | Conduction lengths Presence of stroke Stroke type | Incidence rate of ischemic stroke was higher in aIAB (8.05/1000 person-years vs 3.14; P < 0.0001) Conclusion: aIAB was associated with incident ischemic stroke |
| O'Neal et al[29] | Retrospective cohort | 14625 | 54 ± 5.8 | 6581 males 8044 females | Patients enrolled in the ARIC Study Recruited between 1987 and 1989 | Participants with AF at baseline Missing baseline covariates Missing follow-up data Race other than black or white Black participants from Washington County and Minneapolis | Black Tobacco consumption Diabetes LDL cholesterol level BMI Hypertension Antihypertensive medication | Conduction lengths | Total of 262 aIAB (69 baseline, 193 new) 1929 AF cases were identified aIAB patients presented an AF incidence of 29.8/1000 vs 6.8/1000 of non-aIAB; HR = 3.09 (P < 0.0001) Conclusion: aIAB is a useful marker to identify high risk subjects for developing atrial fibrillation |
| Pirinen et al[41] | Case-control | 690 | 15-49 | 438 males 252 females | Correct diagnosis of IS Part of the Helsinki Young Stroke Study | Unknown stroke date Outpatient treatment only No ECG OR only take on the day of stroke in ER OR no ECG between day of stroke and 14 d after | Coronary heart disease Heart failure Obesity Hypertension Tobacco use Dyslipidemia CHF Preexisting AF #VALUE | Arrhythmia types Conduction lengths Stroke etiology | Most Common ECG abnormalities: T-wave inversion (LVH (14%), prolonged P-wave (13%), prolonged QTc (12%). Most ECG abnormalities in the Stroke Etiology Subgroups: HRCE, LAA and SVD Conclusion: Routine ECG provides useful information for directing the work-up of a young IS patient. In addition to AF, P-terminal force in particular showed a strong association with etiology of high-risk source of cardioembolism |
| Enriquez et al[42] | Prospective cohort | 187 | 67 ± 10.7 | Not reported | Patients with typical atrial flutter (AFI) with no prior history of AF referred for CTI ablation | Patients that had received repeat ablations or did not demonstrate a bidirectional block | Composite of Cardiovascular Disease not reported | Conduction lengths Ejection fraction Holter monitoring | Advanced IAB was detected in 18.2% of patients Left atrium was larger in aIAB (46.2 ± 5.9 mm vs 43.1 ± 6.0 mm; P = 0.01) 35.8% of patients developed new-onset AF |
| Cotter et al[31] | Retrospective cohort | 51 | 17-73 | 28 males 23 females | ILR implanted after unexplained ischemic stroke Brain imaging consistent with embolism Arterial imaging Structural cardiac imaging and rhythm monitoring 50 d of continuous monitoring | TIA Documented cause of stroke before ILR implantation Intrinsic small-vessel disease cause Atheromatosis stenosis > 50% or dissection High-risk cardiac embolic source No AF detected in 24 h - Holter | Not reported | Rhythm monitoring ECG Conduction lengths CHADS2 and CHA2DS2-VASc scores | 25.5% of cases had AF IAB more prevalent in patients with AF (P = 0.02) AF patients larger LA volumes (P = 0.025) Mean AF duration was 6 min Conclusion: In patients with unexplained stroke atrial fibrillation was detected by implantable loop recorders in 25.5%. IAB was an independent predictor of AF |
| Cotter et al[30] | Case-control | 78 | 24-55 | 49 males 29 females | ≤ 55 yr at time of stroke Index cerebral infarct with no cause found CT or MRI imaging, cervical vascular imaging, ECG and rhythm monitoring | Poor quality data | Not reported | Conduction lengths PFO status A-S-C-O Classification | IAB more frequent in cases than controls (40% vs 13%) (P < 0.05) 74.6% of stroke showed PFO (70.3% large) No statistical difference of P-wave length (with vs without PFO) Conclusion: In young patients with unexplained stroke, particularly those with patent foramen ovale atria l dysfunction is a possible mechanism of stroke |
| Ariyarajah et al[43] | Case-control | 66 | 60-87 | 39 males 27 females | Definitive acute or subacute cerebral infarct Probable embolic origin | No 12-lead ECG during 14 d post infarct Non-sinusal rhythm detected in ECG | Hypertension Valvulopathies Cardiomyopathies Tobacco Use Dyslipidemia Diabetes Mellitus Hyper/Hypothyroidism COPD Florid Heart Failure Cardiac Catheterization Myocardial Infection Valvuloplasty Previous strokes/ TIA History of AF/Flutter CAD | Echocardiogram Conduction lengths | 61% IAB prevalence CAD paroxistically more present in control, perhaps due to atherosclerotic origin LA more prevalent in IAB group, with greater LA thrombi (83% vs 0%) Conclusion: IAB could be a risk factor for embolic stroke due to its known sequelae of left atrial dilation and electromechanical dysfunction that predispose to thrombosis |
| Ariyarajah et al[2] | Case-control | 228 | 30-102 | 118 males 110 females | Studied for suspicion of stroke with CT Scan and MRI | No 12-lead ECG during 14 d post infarct | Hypertension Valvulopathies Cardiomyopathies Tobacco Use Dyslipidemia Diabetes Mellitus Hyper/Hypothyroidism COPD Florid Heart Failure Cardiac Catheterization Myocardial Infection Valvuloplasty Previous strokes/ TIA History of AF/Flutter CAD | Conduction lengths Stroke etiology | 61% IAB embolic vs 40% non-embolic (P = 0.006) Hypertension for embolic stroke (P < 0.0001) Conclusion: IAB could be a novel risk for embolic stroke |
| Ariyarajah et al[12] | Prospective cohort | 32 | 66-94 | 15 males 17 females | Saint Vincent Hospital general patients (December 15, 2004 to January 14, 2005) Resting ECG obtained on admission Existing 2-dimensional transthoracic echocardiograms Sinus rhythm | Not reported | Mitral or tricuspid valvular disease Hypertension Coronary artery disease Hyperlipidemia Diabetes mellitus History of AF/Flutter ACEI use Beta-blocker use Statins use | Conduction lengths LA dimension LVEF Cardiovascular events (heart failure, peripheral embolism, transient ischemic attack, stroke, atrial tachyarrhythmias) | Coronary disease was more prevalent in the IAB group Cardiovascular events were overall most significant in IAB, except for stroke, TIA, peripheral arterial embolism and atrial flutter Conclusion: In patients with comparable echocardiographic parameters, IAB remained associated with atrial fibrillation after 15-mo follow-up |
| Lorbar et al[33] | Retrospective cohort | 104 | 22-101 | 58 males 46 females | St Vincent Hospital (January 2000 to December 2001) patients with ICD codes for embolic stroke Diagnosis of embolic ischemic stroke or TIA by a neurologist with or without imaging techniques | Cerebrovascular events non ICD codes Dementia, seizure, hypertensive encephalopathy, subdural hematoma, dizziness, vertigo, psychosis, and headache | Not reported | Conduction lengths ECG patterns | 41% history of AF, or newly diagnosed AF 80% normal sinus rhythm patients showed IAB on concurrent ECG Conclusion: IAB may represent a new factor for stroke |
| Jairat et al[23] | Prospective cohort | 1000 | 24-94 | 585 males 415 females | Saint Vincent Hospital general patients | Not reported | Not reported | Conduction lengths ECG patterns | 32.8% of all patients showed IAB 41.1% of sinus rhythm patients showed IAB Conclusion: Patients with IAB must be followed for atrial enlargement, potential thrombosis, and the onset of atrial fibrillation |
Recognition of Bayés syndrome is not merely an academic issue. It allows selecting high-risk patients for which pharmacological therapy could be beneficial. Open questions remain to be addressed with well-designed clinical trials including whether antiarrhythmic and/or anticoagulant drugs could be used in patients with advanced IAB without atrial tachyarrhythmias to prevent both AF and embolic stroke.
Additional epidemiological studies would be needed to define the possible connection between Bayés syndrome and clinically silent cerebral infarctions, small vessel disease, cognitive impairment of vascular type or dementia.
Bayés syndrome is a poorly recognized cardiac rhythm disorder with important clinical implications. Bayés syndrome is a pre-atrial fibrillation condition and should be considered a novel and important risk factor for cardioembolic stroke and vascular cognitive impairment.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aggarwal A, Petix NR, Petretta M, Said SAM S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Bacharova L, Wagner GS. The time for naming the Interatrial Block Syndrome: Bayes Syndrome. J Electrocardiol. 2015;48:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Ariyarajah V, Puri P, Apiyasawat S, Spodick DH. Interatrial block: a novel risk factor for embolic stroke? Ann Noninvasive Electrocardiol. 2007;12:15-20. [PubMed] [DOI] [Full Text] |
| 3. | Arboix A, Alio J. Acute cardioembolic cerebral infarction: answers to clinical questions. Curr Cardiol Rev. 2012;8:54-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Weir NU. An update on cardioembolic stroke. Postgrad Med J. 2008;84:133-142; quiz 139-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | MacDougall NJ, Amarasinghe S, Muir KW. Secondary prevention of stroke. Expert Rev Cardiovasc Ther. 2009;7:1103-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Khoo CW, Lip GY. Clinical outcomes of acute stroke patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2009;7:371-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Arboix A, Vericat MC, Pujades R, Massons J, García-Eroles L, Oliveres M. Cardioembolic infarction in the Sagrat Cor-Alianza Hospital of Barcelona Stroke Registry. Acta Neurol Scand. 1997;96:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Arboix A, García-Eroles L, Massons J, Oliveres M. Predictive clinical factors of in-hospital mortality in 231 consecutive patients with cardioembolic cerebral infarction. Cerebrovasc Dis. 1998;8:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4767] [Cited by in RCA: 5032] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 10. | Arboix A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J Clin Cases. 2015;3:418-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Chhabra L, Devadoss R, Chaubey VK, Spodick DH. Interatrial block in the modern era. Curr Cardiol Rev. 2014;10:181-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Ariyarajah V, Apiyasawat S, Fernandes J, Kranis M, Spodick DH. Association of atrial fibrillation in patients with interatrial block over prospectively followed controls with comparable echocardiographic parameters. Am J Cardiol. 2007;99:390-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bachmann G. The significance of splitting of the P-wave in the electrocardiogram. Ann Intern Med. 1941;14:1702-1709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Castillo A, Vernant P. [Disorders of intraauricular conduction due to block of Bachman’s bundle]. Arch Mal Coeur Vaiss. 1971;64:1490-1503. [PubMed] |
| 15. | Bayes de Luna AJ. [Block at the auricular level]. Rev Esp Cardiol. 1979;32:5-10. [PubMed] |
| 16. | Bayes de Luna A, Fort de Ribot R, Trilla E, Julia J, Garcia J, Sadurni J, Riba J, Sagues F. Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol. 1985;18:1-13. [PubMed] |
| 17. | Conde D, Baranchuk A. What Cardiologist must know about Bayés Syndrome. Rev Argent Cardiol. 2014;82:237-239. |
| 18. | Conde D, Baranchuk A. [Interatrial block as anatomical-electrical substrate for supraventricular arrhythmias: Bayés syndrome]. Arch Cardiol Mex. 2014;84:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Bayés de Luna A, Cladellas M, Oter R, Torner P, Guindo J, Martí V, Rivera I, Iturralde P. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J. 1988;9:1112-1118. [PubMed] |
| 20. | Bayés de Luna A, Guindo J, Viñolas X, Martinez-Rubio A, Oter R, Bayés-Genís A. Third-degree inter-atrial block and supraventricular tachyarrhythmias. Europace. 1999;1:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Bayés de Luna A, Platonov P, Cosio FG, Cygankiewicz I, Pastore C, Baranowski R, Bayés-Genis A, Guindo J, Viñolas X, Garcia-Niebla J. Interatrial blocks. A separate entity from left atrial enlargement: a consensus report. J Electrocardiol. 2012;45:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 22. | Tse G, Lai ET, Yeo JM, Yan BP. Electrophysiological Mechanisms of Bayés Syndrome: Insights from Clinical and Mouse Studies. Front Physiol. 2016;7:188. [PubMed] [DOI] [Full Text] |
| 23. | Jairath UC, Spodick DH. Exceptional prevalence of interatrial block in a general hospital population. Clin Cardiol. 2001;24:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | O’Neal WT, Zhang ZM, Loehr LR, Chen LY, Alonso A, Soliman EZ. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am J Cardiol. 2016;117:1755-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Mehrzad R, Spodick DH. Interatrial block: a virtual pandemic requiring attention. Iran J Med Sci. 2014;39:84-93. [PubMed] |
| 26. | Bayés de Luna A, Oter MC, Guindo J. Interatrial conduction block with retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmias: influence of preventive antiarrhythmic treatment. Int J Cardiol. 1989;22:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Baranchuk A, Bayes-Genis A. Bayés’ Syndrome. Rev Esp Cardiol (Engl Ed). 2016;69:439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Hughes TM, Worrall BB. Acute interatrial block is a distinct risk factor for ischemic stroke. Neurology. 2016;87:344-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | O’Neal WT, Kamel H, Zhang ZM, Chen LY, Alonso A, Soliman EZ. Advanced interatrial block and ischemic stroke: The Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Cotter PE, Martin PJ, Pugh PJ, Warburton EA, Cheriyan J, Belham M. Increased incidence of interatrial block in younger adults with cryptogenic stroke and patent foramen ovale. Cerebrovasc Dis Extra. 2011;1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology. 2013;80:1546-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 32. | Wu JT, Wang SL, Chu YJ, Long DY, Dong JZ, Fan XW, Yang HT, Duan HY, Yan LJ, Qian P. CHADS2 and CHA2DS2-VASc Scores Predict the Risk of Ischemic Stroke Outcome in Patients with Interatrial Block without Atrial Fibrillation. J Atheroscler Thromb. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Lorbar M, Levrault R, Phadke JG, Spodick DH. Interatrial block as a predictor of embolic stroke. Am J Cardiol. 2005;95:667-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Chhabra L. Importance of P-wave indices in stroke. Int J Cardiol. 2016;203:962-963. [PubMed] [DOI] [Full Text] |
| 35. | Arboix A, Font A, Garro C, García-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psychiatry. 2007;78:1392-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Arboix A, López-Grau M, Casasnovas C, García-Eroles L, Massons J, Balcells M. Clinical study of 39 patients with atypical lacunar syndrome. J Neurol Neurosurg Psychiatry. 2006;77:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Arboix A, Bechich S, Oliveres M, García-Eroles L, Massons J, Targa C. Ischemic stroke of unusual cause: clinical features, etiology and outcome. Eur J Neurol. 2001;8:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Palomeras Soler E, Fossas Felip P, Casado Ruiz V, Cano Orgaz A, Sanz Cartagena P, Muriana Batiste D. The Mataró Stroke Registry: a 10-year registry in a community hospital. Neurologia. 2015;30:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Alvarez-Sabín J. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke. 2007;38:3225-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Martínez-Sellés M, Massó-van Roessel A, Álvarez-García J, García de la Villa B, Cruz-Jentoft AJ, Vidán MT, López Díaz J, Felix Redondo FJ, Durán Guerrero JM, Bayes-Genis A. Interatrial block and atrial arrhythmias in centenarians: Prevalence, associations, and clinical implications. Heart Rhythm. 2016;13:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Pirinen J, Putaala J, Aro AL, Surakka I, Haapaniemi A, Kaste M, Haapaniemi E, Tatlisumak T, Lehto M. Resting 12-lead electrocardiogram reveals high-risk sources of cardioembolism in young adult ischemic stroke. Int J Cardiol. 2015;198:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Enriquez A, Sarrias A, Villuendas R, Ali FS, Conde D, Hopman WM, Redfearn DP, Michael K, Simpson C, De Luna AB. New-onset atrial fibrillation after cavotricuspid isthmus ablation: identification of advanced interatrial block is key. Europace. 2015;17:1289-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Ariyarajah V, Apiyasawat S, Najjar H, Mercado K, Puri P, Spodick DH. Frequency of interatrial block in patients with sinus rhythm hospitalized for stroke and comparison to those without interatrial block. Am J Cardiol. 2007;99:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |