Published online Mar 16, 2017. doi: 10.12998/wjcc.v5.i3.112
Peer-review started: August 11, 2016
First decision: September 12, 2016
Revised: October 11, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: March 16, 2017
Processing time: 214 Days and 13.4 Hours
Lemierre’s syndrome (LS) is an uncommon condition with oropharyngeal infections, internal jugular vein thrombosis, and systemic metastatic septic embolization as the main features. Fusobacterium species, a group of strictly anaerobic Gram negative rod shaped bacteria, are advocated to be the main pathogen involved. We report a case of LS complicated by pulmonary embolism and pulmonary septic emboli that mimicked a neoplastic lung condition. A Medline search revealed 173 case reports of LS associated with internal jugular vein thrombosis that documented the type of microorganism. Data confirmed high prevalence in young males with Gram negative infections (83.2%). Pulmonary embolism was reported in 8.7% of cases mainly described in subjects with Gram positive infections (OR = 9.786; 95%CI: 2.577-37.168, P = 0.001), independently of age and gender. Only four fatal cases were reported. LS is an uncommon condition that could be complicated by pulmonary embolism, especially in subjects with Gram positive infections.
Core tip: We report a case of Lemierre’s syndrome (LS) complicated by pulmonary embolism (PE) that mimicked a neoplastic lung condition. The case was related to previously reported cases in Medline that documented the type of microorganism. We associated PE with LS due to Gram positive infections.
- Citation: De Giorgi A, Fabbian F, Molino C, Misurati E, Tiseo R, Parisi C, Boari B, Manfredini R. Pulmonary embolism and internal jugular vein thrombosis as evocative clues of Lemierre’s syndrome: A case report and review of the literature. World J Clin Cases 2017; 5(3): 112-118
- URL: https://www.wjgnet.com/2307-8960/full/v5/i3/112.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i3.112
Lemierre’s syndrome (LS) is an uncommon condition characterized mainly by oropharyngeal infections complicated with internal jugular vein (IJV) thrombosis and subsequently metastatic infections secondary to septic emboli. This syndrome was first reported by Andrè Lemierre in 1936 in a personal experience describing 20 patients[1].
Primary sites of infection in these patients are the tonsils (palatine tonsils or peritonsillar tissue), pharynx and lower respiratory tract[2]. Fusobacterium represents the most common micro-organism related to this syndrome (about 90% of cases). Fusobacterium spp. are strictly anaerobic Gram-negative rod shaped bacteria, mainly isolated from the oral cavity[3]. The mechanisms underlying virulent clinical conditions are not known, and Fusobacterium is considered a rare cause of head and neck infections[4].
After local proliferation, neck infection is associated with IJV thrombosis and then hematogenous spread to other peripheral organs could happen such as the lung, joints, soft tissue, abdominal parenchyma, and central nervous system[5].
We report a case of LS complicated by pulmonary embolism and pulmonary septic emboli after IJV thrombosis.
A 53-year-old man presented to emergency department because of a history of occipital headache, malaise, hacking cough, chest pain exacerbated by inspiration, and fever for one month. He had a history of smoking, hypertension, hyperuricemia, and gastro-esophageal reflux. His general practitioner treated him unsuccessfully with clarithromycin and ceftriaxone. Blood chemistry panel showed increasing inflammatory indexes, such as white blood cells (WBC) 16.560/mm3, C-reactive protein (CRP) 13.60 mg/dL, and erythrocyte sedimentation rate (ESR) 70 mm. Chest X-ray did not show parenchymal lesions, and either spinal column X-ray or encephalic nuclear magnetic resonance (NMR) was unremarkable.
On admission, the physical examination was unremarkable except that pharyngeal and tonsil hyperemia was detected. He was diagnosed with chronic tonsillitis by an otorhinolaryngologist. Pharyngeal packing with cultural exam identified saprophytic flora. Levofloxacin and nebulizer therapies were prescribed.
Further laboratory tests showed WBC = 11.070/mm3, CRP = 3.70 mg/dL, ESR = 53 mm, fibrinogen = 706 mg/dL, and D-dimer = 773 ng/mL. Immunoglobulin-A was 559 mg/dL. Chest X-ray showed parenchymal and pulmonary consolidation associated with pleural effusion. Bronchoscopy with broncho-alveolar lavage (BAL) including microbiology and cytology was negative.
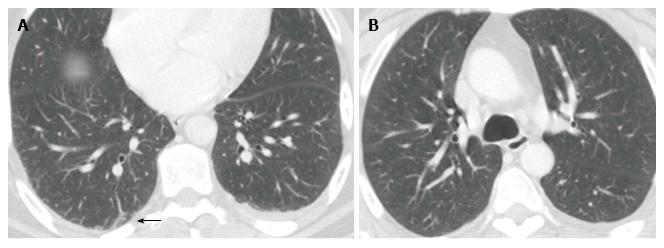
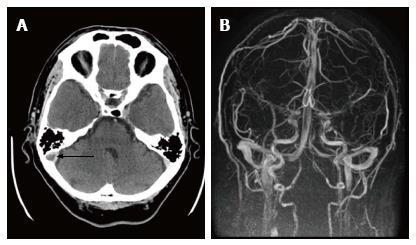
A chest computed tomography (CT) scan showed left pleural effusion, contralateral sub-pleural fibrosis and, above all, an important ovular lesion at the level of medial right lobe measuring 25.7 mm with central cavitation. Further three lesions of 5-6 mm at the superior right lobe, and enlargement of pulmonary hilar lymph nodes were evident (the largest was 13.4 mm). Since these images were suggestive of pulmonary neoplastic lesions (Figure 1A), a brain CT scan was planned. The latter detected a deficit of right sigmoid sinus and bulb of jugular vein filling, which were suggestive of thrombosis of the right jugular vein (Figure 2A). Doppler ultrasonography of upper and lower limbs and echocardiography were negative. A further careful re-evaluation of chest CT supported the hypothesis of septic pulmonary outbreaks, and filling defect in the upper and middle branches of the right pulmonary artery suggested pulmonary embolism (Figure 1B). A diagnosis of LS associated with IJV thrombosis secondary to tonsillitis and pulmonary emboli was made, and low molecular weight heparin (LMWH) was added to levofloxacin. Eleven days later, the patient was discharged in good general conditions. One month after discharge, a cerebral magnetic resonance angiogram (MRA) showed the complete re-canalization of the IJV (Figure 2B).
LS is an oropharyngeal infection complicated with IJV thrombosis and subsequently metastatic infections due to septic emboli[1]. LS represent an uncommon condition, and its prevalence is 0.6-2.3 cases per million population. Mortality rate is 4%-18%[6]. LS incidence is higher in people aged 14-24, and its annual rate is 14.4 cases per million people per year. Mean age of patients is reported to be 18-20 years[6,7]. Male patients seen to be at higher risk, especially in autumn and winter[5].
The most common etiology of LS is infection due to Fusobacterium necrophorum, an anaerobic, non-motile, filamentous and non-spore forming Gram negative rod, which is described in 80% of cases. Several other organisms have been reported, isolated as single pathogen (5% of cases) or in association with Fusobacterium necrophorum (10.1%), such as many bacteria of Bacteroides family, Group B and C Streptococcus, Streptococcus oralis, Staphylococcus epidermidis, Klebsiella pneumoniae, Enterococcus sp., Proteus mirabilis, Eubacterium sp., Eikenella corrodens, lactobacilli and Candida sp. On the other hand, culture results are negative in 12% of cases[7].
The main site of infection is palatine tonsils (87.1% of cases) and it could lead to exudative tonsillitis and peritonsillar tissue ulcer. However, it has been reported that only hyperemia or grey pseudo-membrane could be detected. Moreover, odontogenic infections, mastoiditis, parotitis, sinusitis, otitis, and skin or subcutaneous tissue infection of the head or neck may represent the primary infection site. Finally, the disease could happen even if the appearance of the pharynx was not remarkable[5-7].
Pulmonary embolism is not frequently described in LS. Lesions of the lungs are due to haematogenous spread of bacteria from the IJV, and necrotic cavitary lesions, infiltrates, pleural effusions or empyema, abscesses, pneumo-thoraces, or necrotising mediastinitis have been reported[5].
We performed a Medline literature search to identify papers reporting cases with LS associated with IJV thrombosis. The following search terms were used: “Lemierre syndrome” in combination with “internal jugular vein thrombosis” and “vein thrombosis”. We found that isolation of microorganism was available in 173 cases (Table 1). LS was described more frequently in males (61.3%), aged 25.5 ± 14 years. Gram negative bacteria (84.3%), particularly Fusobacterium spp (76.3%), were related to it. Multiple microorganisms were reported in 8.7% of cases. Complications such as IJV thrombosis, arterial thrombosis, and pulmonary embolism were reported in 71.7%, 2.9% and 8.7% of cases, respectively. Only four fatal cases (2.3%) were described. Univariate analysis (Table 2) showed that pulmonary embolism was more frequent in patients with Gram positive bacteria. This finding was further confirmed by multivariate analysis and we calculated an odd ratio of 9.786 (95%CI: 2.577-37.168, P = 0.001). The relationship was independent from age, gender, and site of thrombosis.
| Ref. | Pathogen |
| Vogel et al, Am J Dis Child 1980 | FN |
| FN | |
| Sinave et al, Medicine (Baltimore) 1989 | FN |
| FN, Staphy. epidermidis | |
| Jones et al, Postgrad Med J 1990 | FN |
| Blok et al, Ned Tijdschr Geneeskd 1993 | FN |
| FN | |
| FN | |
| Ahkee et al, Ann Otol Rhinol Laryngol 1994 | FN |
| Bader-Meuiner et al, Eur J Pediatr 1994 | FN |
| Dykhuizen et al, Eur Respir J 1994 | FN and Bacteroides fragilis |
| Hughes et al, Clin Infect Dis 1994 | Multiple microrganisms |
| Kubota et al, Nihon Kyobu Shikkan Gakkai Zasshi 1994 | FN |
| Alvarez et al, Pediatrics 1995 | FN |
| Gupta et al, Clin Pediatr (Phila) 1995 | FN and Staphy. epidermidis |
| Karanas et al, Ann Plast Surg 1995 | FN |
| Bhagat et al, Infect Dis Clin Pract (Baltim Md) 1996 | Klebsiella pneumoniae |
| De Sena et al, Pediatr Radiol 1996 | FN |
| FN | |
| Harar et al, ORL J Otorhinolaryngol Relat Spec 1996 | FN |
| Lee et al, South Med J 1997 | Strepto. viridans |
| Bouton et al, Rev Med Brux 1998 | FN |
| Dhawan et al, Indian J Pediatr 1998 | Peptostrepto. anaerobius, Bacteroides fragilis, Eikenella corrodens |
| Williams et al, Int J Pediatr Otorhinolaryngol 1998 | Strepto. viridians |
| Strepto. viridians | |
| Gong et al, Eur Radiol 1999 | FN |
| Stokroos et al, Arch Otolaryngol Head Neck Surg 1999 | FN |
| Agarwal et al, J Laryngol Otol 2000 | FN |
| Alifano et al, Ann Thorac Surg 2000 | FS and Propionibacterium |
| Chemlal et al, Presse Med 2000 | Strepto. intermedius |
| Edibam et al, Crit Care Resusc 2000 | FN |
| Gowan et al, Can Respir J 2000 | FN |
| Ockrim et al, J R Soc Med 2000 | FN |
| Shaham et al, Clin Imaging 2000 | FS |
| Abele-Horn et al, Eur J Clin Microbiol Infect Dis 2001 | FN |
| De Vos et al, Neth J Med 2001 | FN |
| Singhal et al, South Med J 2001 | FN |
| Turay et al, Respirology 2001 | FN |
| Chirinos et al, Medicine (Baltimore) 2002 | FN |
| FN | |
| Hoehn et al, Crit Care Med 2002 | FN |
| Hope et al, J Laryngol Otol 2002 | FN |
| Nguyen-Dinh et al, J Neuroradiol 2002 | Strepto. species |
| Boo et al, Ir Med J 2003 | FN |
| Dalamaga et al, Anaerobe 2003 | FN |
| de Lima et al, Pediatr Radiol 2003 | FN |
| Figueras et al, Acta Paediatr 2003 | FN |
| Hodgson et al, Undersea Hyperb Med 2003 | FN |
| Jarmeko et al, CMAJ 2003 | FN |
| Velez et al, J Oral Maxillofac Surg 2003 | FN |
| Williams et al, J Clin Microbiol 2003 | FN |
| Ramirez et al, Pediatrics 2003 | FN |
| FN | |
| FN | |
| FN | |
| FN | |
| Ahad et al, Eye (Lond) 2004 | FN |
| Bentham et al, Pediatr Neurol 2004 | FN |
| Giridharan et al, J Laryngol Otol 2004 | FN |
| FN | |
| FN | |
| Lai et al, N Engl J Med 2004 | FN |
| Litterio et al, Anaerobe 2004 | FN |
| Ritter et al, Ultraschall Med 2004 | FN |
| Aliyu et al, Eur J Clin Microbiol Infect Dis 2005 | FN |
| Charles et al, J Vasc Surg 2005 | FN |
| Dool et al, Eur Arch Otorhinolaryngol 2005 | FN |
| FN | |
| FN | |
| Kuduvalli et al, Acta Anaesthesiol Scand 2005 | FN |
| Libeer et al, Acta Clin Belg 2005 | FN |
| FN and Bacteroides spp | |
| Masterson et al, Int J Pediatr Otorhinolaryngol 2005 | FN |
| Min et al, Angiology 2005 | Staphy. haemolyticus and himinis |
| Morizono et al, Intern Med 2005 | Porphyromonas asaccharolytica |
| Nadkarni et al, J Emerg Med 2005 | FN |
| Nakamura et al, Angiology 2000 | FN |
| Ochoa et al, Acad Emerg Med 2005 | FN |
| Peng et al, J Formos Med Assoc 2005 | FN |
| Rivero Marcotegui et al, An Med Interna 2005 | Mycoplasma pneumoniae |
| Schmid et al, Pediatrics 2005 | FN |
| Shah et al, J Ayub Med Coll Abbottabad 2005 | FN |
| FN | |
| Touitou et al, Eur J Neurol 2006 | FN |
| Venkateswaran et al, Ann Acad Med Singapore 2005 | FS and Bacteroides fragilis |
| Varkey Maramattom et al, Cerebrovasc Dis 2005 | FN |
| Hochmair et al, Wien Klin Wochenschr 2006 | FN |
| Fleskens et al, Ned Tijdschr Geneeskd 2006 | FN |
| FN | |
| Constantin et al, BMC Infect Dis 2006 | FN |
| Ravn et al, Scand J Infect Dis 2006 | FN |
| Morris et al, Ir Med J 2006 | FN |
| Olson et al, Br J Ophthalmol 2006 | FN |
| Park et al, J Bone Joint Surg Br 2006 | FN |
| Perović et al, Acta Med Croatica 2006 | FN |
| Singaporewalla et al, Singapore Med J 2006 | Klebsiella pneumoniae |
| Boga et al, J Thromb Thrombolysis 2007 | Staphy. aureus |
| Brown et al, J Laryngol Otol 2007 | FN |
| Chiu et al, Australas Radiol 2007 | FN |
| Cholette et al, Pediatr Pulmonol 2007 | FN |
| FN | |
| Juárez Escalona et al, Med Oral Patol Oral Cir Bucal 2007 | Strepto. intermedius and Bacteroides fragilis |
| Thompson et al, Infect Dis Obstet Gynecol 2007 | Peptostrepto. anaerobius, Bacteroides fragilis, and Eikenella corrodens |
| Wang et al, Anaesth Intensive Care 2007 | FN |
| Westhout et al, J Neurosurg 2007 | FN |
| Garimorth et al, Wien Klin Wochenschr 2008 | FN |
| Georgopoulos et al, J Laryngol Otol 2008 | FN |
| Kadhiravan et al, J Med Case Rep 2008 | Staphy. aureus |
| Bentley et al, J Emerg Med 2009 | Staphy. aureus |
| Goyal et al, Neurol Sci 2009 | FN |
| Lee et al, J AAPOS 2009 | Strepto. viridans and salivarius |
| Lu et al, J Am Board Fam Med 2009 | FN |
| FS | |
| FS | |
| Takazono et al, Jpn J Infect Dis 2009 | FN |
| van Wissen et al, Blood Coagul Fibrinolysis 2009 | FN |
| FN | |
| Castro-Marín et al, J Emerg Med 2010 | FN |
| Chacko et al, J Laryngol Otol 2010 | FN |
| Herek et al, J Emerg Med 2010 | Staphy. aureus |
| Courtin et al, Ann Fr Anesth Reanim 2010 | FN |
| Bonhoeffer et al, Klin Padiatr 2010 | FN |
| Lim et al, Auris Nasus Larynx 2010 | Staphy. aureus |
| Nakayama et al, Auris Nasus Larynx 2010 | FN |
| Ridgway et al, Am J Otolaryngol 2010 | FN |
| Vargiami et al, Eur J Pediatr 2010 | Abiotrophia defective |
| Vincent et al, J Pediatr 2010 | FN |
| Gülmez et al, Mikrobiyol Bul 2011 | FN |
| Huynh-Moynot et al, Ann Biol Clin (Paris) 2011 | FN |
| Maalikjy Akkawi et al, Neurol Sci 2001 | Klebsiella pneumoniae |
| Naito et al, Nihon Kokyuki Gakkai Zasshi 2011 | FN |
| O'Dwyer et al, Ir J Med Sci 2011 | FN |
| Yamamoto et al, Nihon Rinsho Meneki Gakkai Kaishi 2011 | FN |
| Garbati et al, J Med Case Rep 2012 | Klebsiella pneumoniae |
| Hile et al, J Emerg Med 2012 | Peptococcus anaerobius |
| Kuppalli et al, Lancet Infect Dis 2012 | FN |
| Lee et al, J Microbiol Immunol Infect 2012 | Klebsiella pneumoniae |
| Lim et al, Med J Malaysia 2012 | Klebsiella pneumoniae |
| Teai et al, J Formos Med Assoc 2012 | Klebsiella pneumoniae |
| Teng et al, J Emerg Med 2012 | FN |
| Tsai et al, J Formos Med Assoc 2012 | Klebsiella pneumoniae |
| Abhishek et al, Braz J Infect Dis 2013 | Staphy. aureus |
| Blessing et al, Int J Pediatr Otorhinolaryngol 2013 | FN |
| Khan et al, Indian J Pediatr 2013 | FN |
| Klein et al, Heart Lung 2013 | Mycoplasma pneumoniae |
| Marulasiddappa et al, Indian J Crit Care Med 2013 | Staphylococcus aureus |
| Nguyen et al, Malays J Med Sci 2013 | Klebsiella pneumoniae |
| Phua et al, Int J Angiol 2013 | Klebsiella pneumoniae |
| Righini et al, Head Neck 2014 | FN |
| FN | |
| FN | |
| Strepto. costellatus | |
| Enterococcus faecalis | |
| Strepto. anginosus | |
| Neisseria species | |
| FN | |
| FN | |
| Gunatilake et al, Int J Emerg Med 2014 | Staphy. aureus |
| Asnani et al, J Fam Pract 2014 | FN |
| Galyfos et al, Scand J Infect Dis 2014 | FN |
| Aslanidis et al, Pan Afr Med J 2014 | Candida albicans, Staphy. epidermidis, and Klebsiella pneumonia |
| Karnov et al, Open Forum Infect Dis 2014 | FN |
| Choi et al, Tuberc Respir Dis (Seoul) 2015 | Staphy. epidermidis |
| Chuncharunee et al, Hawaii J Med Public Health 2015 | Klebsiella pneumoniae |
| Croft et al, Respir Med Case Rep 2015 | FN |
| Fischer et al, Infect Dis Rep 2015 | FN |
| He et al, BMJ Case Rep 2015 | FN |
| Kempen et al, Eur Spine J 2015 | Strepto. milleri and FS |
| Prakashchandra et al, J Clin Diagn Res 2015 | FN |
| Oya et al, Intern Med 2015 | FS |
| Takano et al, BMC Res Notes 2015 | FN |
| Wong et al, J Am Board Fam Med 2015 | FN |
| Habert et al, Rev Mal Respir 2016 | FN |
| No pulmonary embolism (n = 158) | Pulmonary embolism (n = 15) | P | |
| Female, n (%) | 61 (38.8) | 6 (38.5) | NS |
| Male, n (%) | 97 (61.2) | 9 (61.5) | |
| Age, (yr) | 25.5 ± 14.1 | 26.2 ± 13.6 | NS |
| Gram positive bacteria, n (%) | 21 (13.3) | 6 (40) | 0.006 |
| Gram negative bacteria, n (%) | 137 (86.7) | 9 (60) | |
| Multiple microrganisms, n (%) | 14 (8.9) | 1 (6.7) | NS |
| Only jugular vein thrombosis, n (%) | 113 (71.5) | 11 (73.3) | NS |
| Arterial thrombosis, n (%) | 5 (3.2) | 0 | NS |
| Fatal cases, n (%) | 4 (2.5) | 0 | NS |
In conclusion, LS is a rare condition that can mimic a neoplastic disease. However, the careful evaluation of clinical evolution should suggest a correct diagnosis. Moreover, the presence of pulmonary embolism represents a serious complication, and should be suspected when infection is due to Gram positive bacteria.
We are indebted to Mrs. Francesca Molinari and Mrs. Cristina Rinaldi, from the University of Ferrara Library Staff, and to Dr. Donato Bragatto, Dr. Claudia Righini, Mrs. Manuela Zappaterra, from the Health Science Library of the Azienda Ospedaliera-Universitaria of Ferrara, for their valuable and precious collaboration.
A 53-year-old man with a history of smoking, hypertension, hyperuricemia, and gastro-esophageal reflux presented with occipital headache, malaise, hacking cough, chest pain exacerbated by inspiration, and fever.
Physical examination showed only pharyngeal and tonsil hyperemia related to chronic tonsillitis in the patient with a history of gastro-esophageal reflux.
Pulmonary infection with slow resolution, pulmonary abscess, and pulmonary neoplasia.
Laboratory work-up showed increased white blood cells, C-reactive protein, and erythrocyte sedimentation rate.
Chest X-ray was negative for parenchymal lesions at first, but then it showed parenchymal and pulmonary consolidation associated with pleural effusion confirmed by a computed tomography scan. Moreover, filling defect in the upper and middle branches of the right pulmonary artery suggestive of pulmonary embolism was detected. A brain computed tomography scan excluded parenchymal lesions, but a deficit of the right sigmoid sinus and bulb of jugular vein filling suggestive of thrombosis of right jugular vein were shown.
Tonsillitis related to Fusobacterium infection complicated with internal jugular vein thrombosis and pulmonary embolism.
The patient was treated with levofloxacin and low molecular weight heparin.
Lemierre’s syndrome is a rare condition characterized by oropharyngeal infection complicated by internal jugular vein thrombosis and pulmonary embolism.
Lemierre’s syndrome could mimic a neoplastic process. A careful follow-up of this condition is necessary.
The paper is well written.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Grignola JC, Lazo-Langner A, Pereira-Vega A, Tarazov PG, Turner AM, Wang HY S- Editor: Qiu S L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Lemierre A. On certain septicemias due to anaerobic organisms. Lancet. 1936;1:701-703. [RCA] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 574] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Karkos PD, Asrani S, Karkos CD, Leong SC, Theochari EG, Alexopoulou TD, Assimakopoulos AD. Lemierre’s syndrome: A systematic review. Laryngoscope. 2009;119:1552-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721-5732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 2018] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 4. | Yusuf E, Halewyck S, Wybo I, Piérard D, Gordts F. Fusobacterium necrophorum and other Fusobacterium spp. isolated from head and neck infections: A 10-year epidemiology study in an academic hospital. Anaerobe. 2015;34:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kuppalli K, Livorsi D, Talati NJ, Osborn M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect Dis. 2012;12:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Chuncharunee A, Khawcharoenporn T. Lemierre’s Syndrome Caused by Klebsiella pneumoniae in a Diabetic Patient: A Case Report and Review of the Literature. Hawaii J Med Public Health. 2015;74:260-266. [PubMed] |
| 7. | Chirinos JA, Lichtstein DM, Garcia J, Tamariz LJ. The evolution of Lemierre syndrome: report of 2 cases and review of the literature. Medicine (Baltimore). 2002;81:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |