INTRODUCTION
Topiramate (TPM) is a broad-spectrum antiepileptic drug (AED) to treat varieties of seizures in adults and children. TPM is recommended as add-on or mono-therapy to treat patients two or more years old with generalized tonic clonic epilepsy or focal epilepsy with or without secondary generalization which are refractory to treatment with other AEDs; and for Lennox-Gastaut syndrome (LGS)[1]. TPM has been approved by the United States Food and Drug Administration (FDA) in combination with phentermine for weight loss[2] and for migraine prevention[3]. TPM has also off-label uses, i.e., not mentioned in patients’ leaflet and/or prescribing information] which include treatment of bipolar disorder[4]; borderline personality disorder[5]; alcoholism[6]; and antipsychotics-induced weight gain[7]; and as a mood stabilizer[8].
TPM has many adverse side effects. Some are very common (> 10% incidence) including dizziness, weight loss, paraesthesia in the face, mouth and extremities (pins and needles which occur in 12%-14% of patients), somnolence, nausea, diarrhea and fatigue. Others are common (1%-10% incidence) including disturbance in attention, memory deficits, amnesia, cognitive disorder, psychomotor slowing, abnormal coordination, tremors, sedation, vomiting, vertigo, tinnitus, dry mouth, abnormalities of taste and abdominal discomfort. However, most of these adverse effects are mild/moderate, transient and related to higher doses and/or rapid dose titration rate. Thus, these side effects can be reduced or prevented by starting TPM at low doses and gradually increasing the dosage[9]. Also, TPM has some rare and serious side effects which necessitate drug withdrawal and replacement by alternative, these include acute angle glaucoma, acute myopia, decreased sweating and increase in body temperature, confusion, speech arrest[10], manifest metabolic acidosis[11] and urolithiasis of clinical importance[12]. Most of these side effects are related to the carbonic anhydrase enzyme inhibition properties of TPM.
Review of the literature shows that AEDs therapy is rarely associated with peripheral neuropathy. Peripheral neuropathy is a rare adverse effect of phenytoin (PHT) as evidence by clinical and experimental studies[13,14]. It had been reported with short-term treatment (hours to weeks) with PHT in toxic[15-18] or non-toxic doses[19-21] and with long-term (≥ 5 years) PHT therapy[22-25]. Peripheral neuropathy had been also reported with therapy with other AEDs as carbamazepine (CBZ)[26-28], phenobarbital (PB)[27], sodium valproate (VPA)[26,27,29,30], gabapentin (GPN)[31], levetiracetam (LEV)[32] and lacosamide (LCM)[33]. There is no previous report for peripheral neuropathy induced by TPM.
CASE REPORT
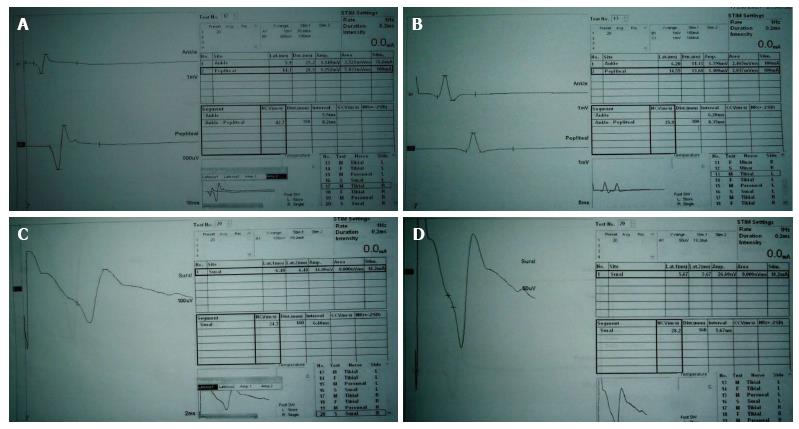
A 37-year-old well-nourished woman presented at the year 2010 with frequent attacks (two or more ictal attacks per week) of generalized tonic clonic convulsions. Clinical, electroencephalography (EEG) and magnetic resonance imaging diagnosis are consistent with idiopathic frontal lobe epilepsy with secondary generalization. The patient has a brother, sister and son with chronic active epilepsy. The patient tried different AEDs as mono- or combined therapies [CBZ and/or VPA, LEV or lamotrigine (LTG)] but with no significant improvement. TPM (100 mg BID) was started (2013) as monotherapy (associated with gradual withdrawal of the other administered AEDs) and the patient became seizure free few months after the start of TPM. The patient experienced some transient side effects which included sense of pins and needles in the face, mouth, body and limbs; myalgia, muscle spasms (cramps) and increased forgetfulness which improved spontaneously within weeks to few months. Laboratory investigations demonstrated hypocalcemia (serum Ca2+ = 7.6 mg/dL). Reassurance of the patient was done and muscle spasms and myalgia disappeared with vitamin D and calcium supplementations. Two years after starting TPM therapy (2015), the patient developed persistent distal numbness in the lower extremities. Neurological examination revealed presence of diminished knee and ankle tendon reflexes, diminished pain and temperature sensation of stocking distribution and decreased vibration perception in the lower limbs. Nerve conduction velocity studies of the median, ulnar, common peroneal, posterior tibial and sural nerves revealed prolonged distal latencies of the tibial nerves (right = 5.9 ms, left = 6.2 ms), reduced their motor conduction velocities (MCVs) (right = 42.7 m/s, left = 35.9 m/s) and amplitudes of their motor action potentials (MAPs) (right = 1.14, 1.25 mV, left = 1.39, 1.10 mV); prolonged distal latencies of sural nerves (right = 6.48 ms, left = 5.67 ms), reduced their sensory conduction velocities (SCVs) (right = 24.7 m/s, left = 28.2 m/s) and amplitudes of their sensory action potentials (SAPs) (right = 14.00 μV, left = 26.6 μV) (Figure 1). The diagnosis of TPM induced peripheral neuropathy was probably suggested after exclusion of the general and common risk factors for the development of peripheral neuropathy which include diabetes, toxins, nutritional disorders (e.g., B-vitamin deficiency) and infectious (e.g., tuberculosis and HIV), connective tissue and metabolic diseases and according to the Naranjo adverse drug reaction (ADR) probability scale (ADR score = 7)[34]. Vitamin B supplementations (thiamine, riboflavin, pyridoxine, cyanocobalamin and folic acid) and anti-oxidants (lipoid acid, primrose oil and vitamin E) were prescribed for the patient for several weeks but no improvement was observed in peripheral neuropathy manifestations. The decision to continue on TPM was discussed with the patient because she is well-controlled (seizure free) on TPM monotherapy after failure to control seizures with other AEDs, no worsening of peripheral neuropathy with time and this side effect was well tolerated by the patient.
Figure 1 Nerve conduction velocity study traces of the right (A) and left tibial (B) nerves and right (C) and left sural (D) nerves show prolonged distal latencies, reduced motor and sensory conduction velocities and reduced motor and sensory action potentials (amplitudes).
This study was conducted according to the principles established in Helsinki and approved by Assiut University Hospital ethics committee. Informed written consent was obtained from the patient to publish the details of her clinical history, laboratory and neurophysiological data.
DISCUSSION
Nearly 12%-14% of patients on TPM commonly experience transient parasthesia in the face, mouth and extremities early during treatment[9] which disappears spontaneously or with the use of potassium therapy[35], however, it may be severe and intolerable in some patients and resulted in discontinuation of TPM[9]. Some evidence suggests that the tendency to cause paresthesia is due to TPM effect on an enzyme called carbonic anhydrase which is an enzyme found in nerve tissue, and probably helps nerve cells talking to one another[35]. However, it seems that another cause of parasthesia may occur with chronic TPM therapy due to the effect of TPM on peripheral nerves. This is the first report of presence of peripheral neuropathy in a patient with epilepsy due to chronic therapy with TPM. Peripheral neuropathy induced by TPM is manifested by parathesia in both lower limbs, decreased ankle jerks, stocking distribution of hypesthesia and delayed distal latencies, reduced nerve conduction velocities of motor and sensory peripheral nerves and reduced amplitudes of motor and sensory action potentials of the peripheral nerves of the lower limbs, indicating demyelinating and axonal peripheral neuropathy. As the patient is seizure free on TPM after several years of ineffective other AEDs therapies, I was unable to do re-challenge testing (stopping and re-staring the treatment to be sure that it was the cause peripheral neuropathy). Only reassurance of the patient was done and no specific treatment was prescribed as the patient has mild paraesthesia and non-progressive peripheral neuropathy.
In general, AEDs are used to treat cortical hyperexcitable states which result in epilepsy and peripheral nerve hyperexcitability which both result in neuropathic pain. Regarding TPM, some experimental and clinical studies demonstrated its efficacy to treat neuropathic pain. Lopes et al[36] demonstrated the antinociceptive effect of oral administered doses of TPM (80 mg/kg) in the models of nociception induced by chemical (formalin) or thermal (hot plate) stimuli. Siniscalchi et al[37] reported complete improvement of idiopathic glossodynia in a 65-year-old woman with 4 mo history of glossodynia with TPM after failure of CBZ or GPN. Glossodynia is a painful sensation in the mouth, throat and especially the tongue due to altered excitability in the trigeminal nociceptive pathway at peripheral and/or central nervous system levels. In another study, Siniscalchi et al[38] reported improvement of dysesthetic pain with TPM (150 mg/d within 8 mo) in a 42-year-old woman with 8 years history of multiple sclerosis. Erdoğan et al[39] observed a significant decrease in the strength duration time constant (which provides an indirect idea about the persistent, paranodal sodium channels and may indirectly reflects the peripheral nerve excitability) but did not observe significant affection of median nerve motor and sensory conduction parameters after the initiation of TPM for 4 wk, reflecting a reduction in the peripheral nerve excitability induced by TPM.
In the literature, peripheral neuropathy had been reported as a rare adverse effect with short-term treatment (hours to weeks) with PHT in toxic[15-18] or non-toxic doses[19-21] and with long-term (months to years) PHT therapy[22-25]. Acute peripheral neuropathy induced by PHT is very rare and reversible side effect[17,19-24]. Hopf et al[19] reported slight but significant reduction in the mean ulnar nerve conduction velocity in 13 patients after the intake of 500-600 mg PHT per day for a week which was not correlated with serum PHT levels. Lovelace and Horwitz[40] reported decrease in motor and sensory conduction velocities of the peripheral nerves without any symptoms (occult) during PHT administration among patients with epilepsy. Birket-Smith and Krogh[15] reported peripheral neuropathy with PHT level more than 20 μg/mL, however, no correlation was observed between the clinical toxicity severity and the degree of conduction velocity abnormalities. Meienberg et al[17] reported acute severe mainly motor polyneuropathy in the legs and cerebellar symptoms after treatment of a 34-year-old epileptic male with high doses PHT to control status epilepticus although this patient was treated for more than ten years with an average of 300 mg PHT and 200-300 mg phenobarbital (PB) daily. Fujiwara et al[24] reported prolonged distal latency of the tibial nerve and decreased mixed nerve action-potential amplitudes of the posterior tibial and median nerves. Wessely et al[20] reported an axonal polyneuropathy with minimal reduction in motor nerve conduction and a considerable extension of distal latency and diminution of compound action potential in 5 patients who were treated with long-term PHT for epilepsy. In 4 cases, the symptoms appeared following treatment of status epilepticus with additional PHT medication. All patients had acute symptomatic psychosis, diffuse slowing of the curves in the EEG and cerebellar signs and two of them additionally complained of objective polyneuropathy. Nerve biopsy in one patient showed concentric lamellar bodies coming from the axon with intact myelin sheaths. Ramirez et al[18] reported a 47-year-old man with clinical and electrophysiological signs of peripheral neuropathy after 30 years treatment with PHT (300 mg/d, the blood levels were 31-38 μg/mL). A sural nerve biopsy showed loss of large myelinated nerve fibers and non-random clustered distribution of segmental demyelination, remyelination and axonal shrinkage. Clinical and electrophysiological improvement was observed within 16 mo of PHT withdrawal. Yoshikawa et al[21] reported an 18-year-old girl who developed distal lower extremity paresthesia in a stocking distribution, motor weakness, absent Achilles tendon reflexes, slightly reduced sensory conduction velocities and mild prolongation of distal latencies in the lower extremities just few hours after the administration of PHT to control epilepsy. Discontinuation of PHT resulted in gradual disappearance of the symptoms and returning of the distal latencies and sensory conduction velocities to normal. Le Quesne et al[14] demonstrated acute slowing of motor nerve conduction velocity in guinea pigs after only 3-4 d of PHT administration. Furthermore long-term PHT administration can cause of peripheral neuropathy which is more frequent than acute forms. Eisen et al[22] reported peripheral neuropathy with the use of PHT which was correlated with PHT level. Chokroverty and Sayeed[16] reported significant reduction in the mean motor conduction velocity of posterior tibial nerves of epileptic patients treated with PHT for more than 10 years or in patients with serum PHT level above 20 μg/mL. Dobkin[23] reported dysesthesia and sensory and reflex loss in the legs in a patient treated for seizures with PHT in the therapeutic range for one year. Discontinuation of PHT resulted in resolution of peripheral neuropathy. Mochizuki et al[25] reported slowed motor conduction velocities of the ulnar (33.3%) and posterior tibial nerves (23.8%), followed by slowed sensory conduction velocities of the sural nerves (20%), lowered H/M ratio (14.3%), and slowed motor conduction velocities of the peroneal (14.3%) and median (14.2%) nerves in children with epilepsy. The authors observed significant correlations between the total dosage and duration of therapy with PHT and the reduction of motor conduction velocity in the posterior tibial nerve.
Peripheral neuropathy had been also reported with other AEDs therapy as CBZ[26-28], PB[27], VPA[26,29,30], GPN[31], LEV[32] and LCM[33]. A review of the literature showed that reflex sympathetic dystrophy (RSD) is precipitated by PB in 10%-30% of cases[41,42]. RSD syndrome is clinically characterized by pain and edema of one or more extremities, trophic skin changes and vasomotor instability. Swift et al[29] reported that 16.7% of epileptic patients may develop peripheral neuropathy with different AEDs which is characterized by stocking hypesthesia, reduced Achilles reflexes, slowing of peroneal and sural nerve conduction velocities and prolonged or absent H reflexes and F responses. Geraldini et al[26] reported slowing of the peroneal and median motor nerve conduction velocities and median sensory nerve conduction velocities with CBZ, PB and PHT. Significant correlation was identified between the slowing of the conduction velocity and the daily dose of CBZ but not its serum drug level or duration of treatment. In the study done by Bono et al[27] on 141 adult patients treated for less than 6 mo with standard daily doses of the commonest AEDs, the authors reported that 53% of patients had one or more symptoms of polyneuropathy (paresthesias being the most common complaint). The neurologic examination was abnormal in 32%. Electrophysiologic findings in two or more separate nerves were abnormal in 77 patients (54.6%); of these, 27 (19.1%) had abnormal neurologic findings and 21 (14.9%) also had symptoms of polyneuropathy. Sensory functions were the most frequently impaired. Axonal damage with secondary myelin changes was noted in sural nerve biopsies of patients on CBZ, PB and PHT. A correlation was noted between polyneuropathy and combined therapy with two or more AEDs. Gould[31] reported a 58-year-old man who developed a painful polyneuropathy while being treated with GPN although GPN is considered an effective treatment for neuropathic pain syndromes. Kapoor et al[32] reported a case of polyneuropathy induced by LEV which improved with discontinuation of LEV. Boylu et al[28] reported mild prolongation in the distal latency of median sensory, ulnar sensory and sural nerves with diminished nerve conduction velocities with chronic CBZ therapy but not with VPA, oxcarbazepine (OXC) or TPM. Marusic et al[30] reported a 26-year-old man with weakness of flexion and abduction of the right arm and loss of sensation in the skin over the lateral upper right arm and reduced amplitude and prolonged latencies in the right axillary nerve because of a suicide attempt with VPA overdose (serum VPA level = 2896 μmol/L; therapeutic range = 350-690 μmol/L). In an experimental study, Zafeiridou et al[33] observed a differential effect for LCM, PHT and TPM on peripheral nerve excitability. The authors reported inhibition of compound action potential of the sciatic nerve of an adult rat after 48 h period of LCM exposure at concentrations higher than the therapeutic level (> 25 μg/mL). An acute and immediate increment of the latency and decrement of the amplitude of the nerve compound action potential were observed at LCM concentrations of 62.57-125.15 μg/mL. However, in contrast to LCM, PHT resulted in an acute decrement in the amplitude of the nerve compound action potential as well as an increment in the latency of the compound action potential even at sub-therapeutic levels (5 μg/mL). Reduced compound motor action potential amplitude was also observed with TPM at concentration of 33.94 μg/mL (supra-therapeutic).
The mechanism (pathogenesis) of PHT induced peripheral neuropathy is not well known. Experimental studies demonstrated a depressant effect of PHT on peripheral nerves[13] which has been attributed to the direct toxic effect of the drug on peripheral nerves and/or due to blockage of sodium channels which is its main anticonvulsant mechanism of action. Korey[43] demonstrated an inhibitory effect of PHT on the giant axon of the squid which was made hyperexcitable by low calcium and magnesium levels. Eisen et al[22] reported a primary axonal shrinkage and secondary demyelination with PHT. Long et al[44] and Hansen et al[45] demonstrated that peripheral neuropathy induced by PHT was related to the subnormal serum folate in association with megaloblastic anemia. We suggest that peripheral neuropathy induced by TPM may be related to its anticonvulsant mechanism of action which is multifactorial and involve blockade of voltage-dependent sodium channels (similar to PHT); inhibition of high-voltage-activated calcium channels; potentiation of GABAergic transmission through GABA-A receptors; inhibition of excitatory pathways through an action at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) AMPA/kainate receptors sites and inhibition of carbonic anhydrase isoenzymes[1].
We report a patient with peripheral neuropathy after chronic therapeutic dose of TPM. However, this adverse effect was mild, static and tolerated by the patient and did not disappear with vitamin B supplementations. There is also a need for experimental and clinical studies to identify the effect of TPM on peripheral nerves and to identify the mechanism(s) of TPM induced peripheral neuropathy.
COMMENTS
Case characteristics
A 37-year-old woman presented with frontal lobe epilepsy with secondary generalization which was intractable to different antiepileptic medications as mono- or combined therapy. Topiramate (TPM) monotherapy significantly controlled the patient’s seizures. After two years of therapy with TPM, the patient developed paresthesia, diminished Achilles tendon reflexes, stocking hypesthesia and delayed distal latencies, reduced conduction velocities and amplitudes of action potentials of posterior tibial and sural nerves.
Clinical diagnosis
Peripheral neuropathy probably induced by long-term TPM therapy.
Differential diagnosis
Other causes of peripheral neuropathy which include diabetes, toxins, nutritional disorders (e.g., B-vitamin deficiency) and infectious, connective tissue and metabolic diseases.
Laboratory diagnosis
Demyelinating and axonal peripheral neuropathy of the tibial and sural nerves.
Treatment
Reassurance of the patient and continue therapy with TPM because the patient is well-controlled (seizure free) on TPM therapy, no worsening of the course of peripheral neuropathy with time and this side effect was well tolerated by the patient.
Related reports
Peripheral neuropathy has been reported as adverse side effect of some antiepileptic drugs (AEDs) including phenytoin, phenobarbital, carbamazepine, valproate, gabapentin, levetiracetam and lacosamide. Most of case reports in the literature are peripheral neuropathy induced by short-term or long-term treatment with phenytoin. There is no previous report for TPM induced peripheral neuropathy.
Term explanation
Peripheral neuropathy is a rare adverse effect of short- or long-term use of AEDs. The risks for peripheral neuropathy induced by AEDs include the high drug doses, high drug serum levels and longer duration of therapy. Some of AEDs may induce acute or severe peripheral neuropathy which necessitates drug withdrawal and use of alternative. There is no previous report of TPM induced peripheral neuropathy. This study is the first report of peripheral neuropathy which is most probably induced by long-term use of TPM.
Experiences and lessons
According to the Naranjo adverse drug reaction probability scale, it seems that chronic TPM therapy is the most probable cause of patient’s neuropathy. Peripheral neuropathy induced by TPM is mild/moderate in severity, non-progressive and not bothersome to patients and may not necessitate drug discontinuation.
Peer-review
The presented case is interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chogtu B, Dumitrascu DL, Masocha W S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ