Published online Dec 16, 2017. doi: 10.12998/wjcc.v5.i12.412
Peer-review started: August 8, 2017
First decision: September 20, 2017
Revised: September 27, 2017
Accepted: October 29, 2017
Article in press: October 29, 2017
Published online: December 16, 2017
Processing time: 124 Days and 19.7 Hours
To examine the effect of Helicobacter pylori (H. pylori) eradication therapy on the extra-gastrointestinal factors in elderly patients by a before-after observational study in community medicine.
Medical records (1 May 2013-31 January 2014) of 130 patients who underwent H. pylori eradication therapy with 2-year after-eradication observation in our institute were reviewed. Data on sex; age; body weight; body mass index (BMI); mean corpuscular volume (MCV); total protein; low-density lipoprotein cholesterol, triglyceride, haemoglobin A1c and haemoglobin levels and gastric hyperplastic polyps (GHPs) at eradication was extracted. Two-year after-eradication change in data was analysed by paired-sample t-test; relationship between GHPs and subclinical iron deficiency anaemia (IDA) improvement was evaluated.
The mean patient age (median, interquartile range) at eradication was 69.6 (71.5, 64-77) years. Paired-sample t-tests showed that body weight, BMI and MCV increased by 0.52 kg (P = 0.018), 0.25 kg/m2 (P = 0.006) and 0.83 fL (P < 0.001), respectively. The nonparametric Mann-Whitney test showed no significant difference in the change rate of MCV after eradication between the groups with and without GHPs (P = 0.892).
H. pylori eradication therapy prevented weight loss and subclinical IDA in elderly individuals. GHPs were not associated with subclinical IDA.
Core tip: The effect of Helicobacter pylori (H. pylori) eradication therapy on the extra-gastrointestinal factors in elderly patients was focused in this study. H. pylori eradication therapy prevented weight loss and subclinical iron deficiency anaemia (IDA) in elderly individuals. Gastric hyperplastic polyps were not associated with subclinical IDA. The results obtained in this study will help physician to treat elderly patients in community-based medicine.
- Citation: Maruyama M, Kamimura K, Hoshiyama A, Hoshiyama K, Hoshiyama M, Hoshiyama Y, Terai S. Effect of Helicobacter pylori eradication on elder cases: Observational study in community-based medicine. World J Clin Cases 2017; 5(12): 412-418
- URL: https://www.wjgnet.com/2307-8960/full/v5/i12/412.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i12.412
Helicobacter pylori (H. pylori) infection affects many extra-gastrointestinal symptoms and diseases, including iron deficiency anaemia (IDA), obesity, diabetes mellitus and hyperlipidemia[1,2]. Although major population surveys and meta-analysis have revealed an increased risk for IDA in addition to a strong evidence for the efficacy of H. pylori eradication for the treatment of unexplained IDA, the relationship between H. pylori infection and prevalence of other extra-gastrointestinal tract diseases is unclear. The influence of H. pylori pathogenicity is currently unknown, particularly in elderly individuals[1,3-6]. In addition, the underlying mechanism of H. pylori-related IDA is still unclear[7,8].
H. pylori eradication therapy for patients with peptic ulcer is associated with gain of body weight[9,10]. The relationship between H. pylori infection and overweight is unclear, even in large-scale epidemiological studies[11-14]. However, this increase might related to the recovery of peptic ulcer and chronic inflammation. On the other hand, because of previously reported inconsistent results, the cause-and-effect relationship between H. pylori infection and metabolic disease is also ambiguous, and there are few reports on elderly individuals[2,15-19]. Because the development of an aging society may be upcoming event in the near future, the effect of H. pylori eradication therapy on the extra-gastrointestinal organs in elderly individuals should be investigated.
Therefore, the purpose of this observational study was to examine the effects of H. pylori eradication in elderly individuals on systemic conditions including body weight, biochemical results, and manifestations of clinical or subclinical anaemia comparing data between before-eradication and 2 years after H. pylori eradication. We have also compared rates of IDA improvement in chronic gastritis with and without gastric hyperplastic polyp (GHP) to investigate the relationship between GHP and H. pylori-related IDA.
This was an observational before-after study in which the case group included 130 individuals who were continuously treated with medications for chronic diseases, such as essential hypertension, hyperlipidemia and/or diabetes mellitus. They were all diagnosed with H. pylori-infected chronic gastritis by routine esophagogastroduodenoscopy (EGD) and the rapid urease test at Kashiwazaki Central Hospital between 1 May 2013 and 31 January 2014.
The patient was considered to be eligible when fulfilled the following inclusion criteria: (1) H. pylori eradication therapy was successful and was followed by the urea breath test; and (2) the patient had been measured/tested for body weight; body mass index (BMI); mean corpuscular volume (MCV); total protein (TP) and low-density lipoprotein cholesterol (LDL-C), triglyceride (TG), haemoglobin (Hb) and haemoglobin A1c (HbA1c) levels at two time points: Before and 2 years after H. pylori eradication therapy was completed. However, we included patients with some missing measurement values and as elderly if older than 65 years old. We excluded patients with mucosal breaking lesions, such as gastric cancer or peptic ulcers, history of gastrointestinal surgery, and the other diseases might cause anemia. This study was approved by the institutional review board of Kashiwazaki Central Hospital. Written informed consent was obtained from all patients, and the study was conducted in accordance with the ethical guidance of the 1975 Declaration of Helsinki.
To identify differences in a patient between two time points, a paired-sample t-test was performed. When there were ≤ 30 cases, a Wilcoxon signed test was performed. For continuous variables, two-group comparisons, such as Hb and MCV, were performed using the nonparametric Mann-Whitney test because assumptions of normality of the distribution were not verified. We excluded the patients with missing data in each group before analysis. The threshold for significance was P < 0.05. In all statistical analysis, we used EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics[20]. The results for changes in variable before and after H. pylori eradication are presented as the mean ± SD.
Between 1 May 2013 and 31 January 2014, 228 patients were diagnosed as having H. pylori-infected chronic gastritis by EGD and the rapid urease test were included. The patients who had been diagnosed as having gastric cancer (n = 3), gastric ulcer (n = 16), duodenal ulcer (n = 20) and gastro-duodenal ulcer (n = 7) who could not be followed up for 2 years after H. pylori eradication (n = 52), a total of 98 patients, were excluded from the initial 228 patients with H. pylori-infected chronic gastritis. Finally, a total of 130 patients [mean age, 69.6 years; median age, 71.5 (interquartile range, 64–77 years); 52 (40%) males] were analysed in the study. No patients showed re-infection of H. pylori after the eradication.
The effect of H. pylori eradication therapy on various physiological factors was carefully examined comparing the value before and after the therapy in all 130 elderly patients with the interval of 2 years for each (Table 1). The body weight increased from a mean ± SD of 57.3 ± 10.4 kg before H. pylori eradication to 58.2 ± 10.3 kg 2 years after H. pylori eradication (P = 0.018). In addition, BMI increased from 23.4 ± 3.1 before H. pylori eradication to 23.7 ± 3.0 2 years after H. pylori eradication (P = 0.006). MCV increased from 89.2 ± 4.9 fL before H. pylori eradication to 90.0 ± 4.4 fL 2 years after H. pylori eradication (P < 0.001) whereas no significant changes were seen in the value of Hb (P = 0.84). The paired-sample t-test showed no significant differences in other measurements including TP, LDL-C, TG, and HbA1c, before and 2 years after H. pylori eradication (Table 1).
| Variable | Subjects | Missing | Pre-eradication mean (SD) | Post-eradication mean (SD) | Mean difference | 95%CI | P value |
| Body weight (kg) | 124 | 6 | 57.3 (10.4) | 58.2 (10.3) | 0.52 | 0.09-0.94 | 0.018 |
| BMI (kg/m2) | 121 | 9 | 23.4 (3.1) | 23.7 (3.0) | 0.25 | 0.074-0.42 | 0.006 |
| Hb (g/dL) | 115 | 15 | 13.8 (1.4) | 13.8 (1.3) | 0.018 | -0.35 | 0.84 |
| MCV (fL) | 113 | 17 | 89.2 (4.9) | 90 (4.4) | 0.83 | 0.32-1.34 | < 0.001 |
| TP (g/dL) | 90 | 40 | 7.4 (0.5) | 7.5 (0.4) | 0.024 | -0.168 | 0.56 |
| LDL-C (mg/dL) | 107 | 23 | 114.3 (23.9) | 116.2 (25.0) | 1.20 | -8.12 | 0.55 |
| TG (mg/dL) | 107 | 23 | 122 (70.5) | 126.6 (77.1) | 6.81 | -22.59 | 0.23 |
| HbA1c (%) | 42 | 88 | 6.2 (0.81) | 6.3 (0.75) | 0.057 | -0.305 | 0.45 |
The patients older than 65 years old were considered to be elderly and the factors affected by the H. pylori eradication treatment have been carefully assessed by the subgroup analyses (Table 2). In the group of patients ≥ 65 years (n = 97), BMI increased from 23.6 ± 3.0 before H. pylori eradication to 23.8 ± 3.1 2 years after H. pylori eradication (P = 0.045). MCV increased from 89.2 ± 5.3 fL before H. pylori eradication to 90.1 ± 4.7 fL 2 years after H. pylori eradication (P = 0.0017) whereas no significant changes were seen in the value of Hb (P = 0.84). There were no significant differences in other measurements in the group of patients ≥ 65 years (Table 2).
| Variable | Subjects | Missing | Pre-eradication mean (SD) | Post-eradication mean (SD) | Mean difference | 95%CI | P value |
| Body weight (kg) | 92 | 5 | 57.1 (10.4) | 57.8 (10.3) | 0.41 | -1.017 | 0.12 |
| BMI (kg/m2) | 90 | 7 | 23.6 (3.0) | 23.8 (3.1) | 0.21 | -0.4159 | 0.045 |
| Hb (g/dL) | 85 | 12 | 13.7 (1.5) | 13.7 (1.3) | -0.02 | -0.4 | 0.84 |
| MCV (fL) | 85 | 12 | 89.2 (5.3) | 90.1 (4.7) | 0.95 | -1.17 | 0.0017 |
| TP (g/dL) | 66 | 31 | 7.5 (0.5) | 7.5 (0.4) | 0.011 | -0.216 | 0.84 |
| LDL-C (mg/dL) | 77 | 20 | 111.9 (21.2) | 114.2 (24.8) | 1.25 | -9.65 | 0.61 |
| TG (mg/dL) | 77 | 20 | 116.7 (56.2) | 112.6 (44.8) | -1.68 | -20.35 | 0.74 |
| HbA1c (%) | 30 | 67 | 6.4 (0.8) | 6.4 (0.7) | 0.013 | -0.35 | 0.88 |
These results suggest that the H. pylori eradication contribute to maintain the BMI avoiding the loss of body weight, and to recovery from subclinical IDA caused by the chronic inflammation in the stomach. In addition, even with the 2 years period of the study, no significant changes were seen in the various nutritional factors, indicating that the better digestion, absorption, after the eradication therapy.
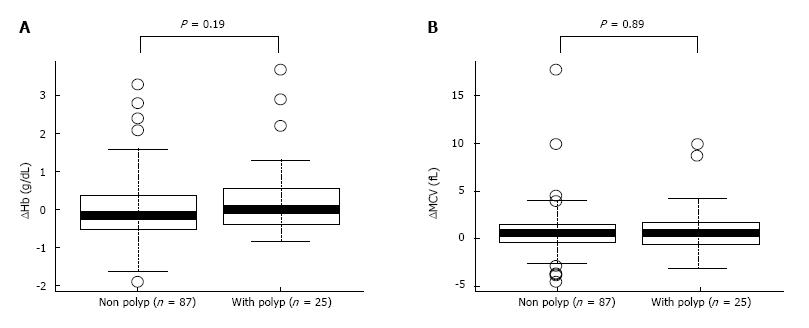
To determine the effect of eradication on anaemia, level of Hb was carefully assessed in the patients (Table 3). Although the patients with Hb levels < 12.5 g/dL before H. pylori eradication increased from 11.5 ± 0.86 g/dL to 12.3 ± 0.99 g/dL at 2 years after H. pylori eradication (P = 0.017), paired-sample t-tests showed no significant difference in Hb levels before and 2 years after H. pylori eradication in the group with Hb ≥ 12.5 g/dL (Table 3). In addition, to examine whether the rates of IDA improvement in chronic gastritis is related to the existence of GHP, the level of improvement of Hb and MCV values before and after the eradication were compared (Figure 1). The nonparametric Mann-Whitney test showed no significant increase in Hb levels and MCV (P = 0.89) from before to 2 years after H. pylori eradication (P = 0.19) between the groups with and without GHP (Figure 1) and its size. These results indicate that the improving tendency of anaemia after H. pylori eradication did not correlate with the presence of GHP or its size.
| Variable | Subjects | Missing | Pre-eradication mean (SD) | Post-eradication mean (SD) | Mean difference | 95%CI | P value |
| Less than Hb 12.5 g/dL | 19 | 1 | 11.5 (0.7) | 12.3 (1.0) | 0.85 | 0.22-1.48 | 0.017 |
| More over Hb 12.5 g/dL | 96 | 11 | 14.2 (1.1) | 14.1 (1.2) | -0.15 | -0.1592 | 0.064 |
Our study showed that H. pylori eradication therapy for elderly patients with chronic gastritis increased BMI and MCV, 2 years as a result of successful H. pylori eradication. The level of MCV has been considered as one of the marker of subclinical IDA and its recovery reflect the improvement of IDA[21]. Previous studies have shown similar results in patients with anaemia whose Hb significantly improved after H. pylori eradication[1,3-6]. There was no difference in the rate of increase in MCV (improvement in IDA) between groups with and without GHP. This finding suggests that GHP is not involved in an anaemic improvement pathway after H. pylori eradication.
It is known that the proportion of individuals with BMI > 30 generally increases up to the age of 60 years, and BMI tends to decrease after the age of 61 years[22]. In addition, the body weight loss in elderly individuals is a predictive factor for death, mildly obese individuals have the lowest mortality rate[23,24]. It might be related to the recently established concept of “Frailty”, a risk factor for falls, disability, hospitalization and mortality during old age. It is defined by the following criteria: Unintentional weight loss, self-reported exhaustion, weakness (grip strength), slow walking speed and low physical activity[25] and energy and protein support is recommended to treat the condition[26]. Interestingly, in our study, we found that elderly patients gained weight after H. pylori eradication. This result was inconsistent with the general tendency towards body weight loss in the elderly population and suggested that the effect of H. pylori eradication on preventing body weight loss or increase. The mechanisms might include, improvement of gastro-duodenal inflammation, ulcerative lesions, etc., as well as decrease of serum level of leptin which plays a crucial role to regulate food intake and energy expenditure[11,27]. Thus, we infer that H. pylori eradication therapy for elderly patients with H. pylori-infected chronic gastritis may be an effective therapy for prevention of weight loss in elderly individuals.
Our results are consistent with those of previous studies showing improvement of anaemia after H. pylori eradication therapy in elderly individuals[1,3-6]. Our study also showed that MCV increased after H. pylori eradication in the total study population as well as in the elderly patient group. However, presence of GHP was not related to the increase in the MCV rate. An important finding from previous study is that 80% of GHP disappeared after H. pylori eradication therapy within an average of 7.1 mo[28]. A recent report suggested that H. pylori-related IDA was associated with several factors in patients with GHP and nodular gastritis[29]. Bleeding from GHP is assumed to be the cause of H. pylori-related IDA. However, a previous study showed that even faecal occult blood-negative patients may be anaemic[30]. In addition, the mechanism might not be applicable to nodular gastritis. A recent study suggested that the cause of anaemia in patients with GHP is not bleeding from GHP but rather a decrease in iron absorption caused by a low-acid state[26]. Therefore, our results provide some support for the hypothesis that the improvement of H. pylori-related IDA is caused by an underlying mechanism other than GHP deletion.
One plausible reason for the finding of no significant changes in TP, TG, LDL-C and HbA1c levels is the presumed administration of statins and/or antidiabetic drugs to the patients. A previous report showed that serum total cholesterol levels did not change after H. pylori eradication[11]. Therefore, our results may be consistent with these previous findings.
A limitation of our study, however, is that although previous studies have shown that diabetes was exacerbated by H. pylori infection[17-19], our findings suggest no exacerbation or improvement of diabetes by eradication was because of strict management by a diabetologist in our hospital. In addition, the power of this study was limited because of the small number of participants and patients with subclinical IDA, of the single-centre analysis and of the retrospective-observational study design. Therefore, future larger, ad hoc, and better designed prospective studies are essential to confirm the effect of H. pylori eradication on systemic conditions by monitoring symptoms, medical history, and laboratory exams comparing with cases failed for the eradication.
In conclusion, our findings suggest that an increase in MCV is associated with body weight gain and improvement of subclinical IDA after H. pylori eradication in elderly patients with chronic gastritis. The tendency for subclinical IDA to improve after H. pylori eradication did not correlate with the presence of GHP. In addition, even with the 2 years period of the study, no significant changes were seen in the various nutritional factors, indicating that the better digestion, absorption, after the eradication therapy. For the future perspective, as the development of an aging society may be upcoming event in the near future, H. pylori eradication therapy may be a useful approach for preventing weight loss and frailty in elderly individuals to keep their quality of life and health.
The relationship between Helicobacter pylori (H. pylori) infection and various extra-gastrointestinal symptoms, including obesity, diabetes mellitus and hyperlipidemia have been reported.
Although major population surveys and meta-analysis have suggested an increased risk for iron deficiency anaemia (IDA), however the relationship between H. pylori infection/its eradication on IDA and other extra-gastrointestinal tract diseases has not been clarified, especially in elderly patients.
This study was aimed to examine the effect of H. pylori eradication therapy on the extra-gastrointestinal factors in elderly patients by a before-after observational study in community medicine.
Medical records (1 May 2013-31 January 2014) of 130 patients who underwent H. pylori eradication therapy with 2-year after-eradication observation in our institute were reviewed. Data on sex; age; body weight; body mass index (BMI); mean corpuscular volume (MCV); total protein; low-density lipoprotein cholesterol, triglyceride, haemoglobin A1c and haemoglobin levels and gastric hyperplastic polyps (GHPs) at eradication was extracted. Two-year after-eradication change in data was analysed by paired-sample t-test; relationship between GHPs and subclinical IDA improvement was evaluated.
The mean patient age (median, interquartile range) at eradication was 69.6 (71.5, 64-77) years. Paired-sample t-tests showed that body weight, BMI and MCV increased by 0.52 kg (P = 0.018), 0.25 kg/m2 (P = 0.006) and 0.83 fL (P < 0.001), respectively. The nonparametric Mann-Whitney test showed no significant difference in the change rate of MCV after eradication between the groups with and without GHPs (P = 0.892).
H. pylori eradication therapy prevented weight loss and subclinical IDA in elderly individuals, therefore, the eradication should be considered even for those elder patients.
For the future perspective, as the development of an aging society may be upcoming event in the near future, H. pylori eradication therapy may be a useful approach for preventing weight loss and frailty in elderly individuals to keep their quality of life and health.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Huang SP, Mehdi I, Milone M, Shrestha BM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148:719-731.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 3. | Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta-analysis. Helicobacter. 2008;13:323-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Yuan W, Li Yumin, Yang Kehu, Ma Bin, Guan Quanlin, Wang D, Yang L. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45:665-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Huang X, Qu X, Yan W, Huang Y, Cai M, Hu B, Wu L, Lin H, Chen Z, Zhu C. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori. Postgrad Med J. 2010;86:272-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Monzón H, Forné M, Esteve M, Rosinach M, Loras C, Espinós JC, Viver JM, Salas A, Fernández-Bañares F. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J Gastroenterol. 2013;19:4166-4171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 7. | Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis. 2003;35:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Barabino A. Helicobacter pylori-related iron deficiency anemia: a review. Helicobacter. 2002;7:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Fujiwara Y, Higuchi K, Arafa UA, Uchida T, Tominaga K, Watanabe T, Arakawa T. Long-term effect of Helicobacter pylori eradication on quality of life, body mass index, and newly developed diseases in Japanese patients with peptic ulcer disease. Hepatogastroenterology. 2002;49:1298-1302. [PubMed] |
| 10. | Kamada T, Hata J, Kusunoki H, Ito M, Tanaka S, Kawamura Y, Chayama K, Haruma K. Eradication of Helicobacter pylori increases the incidence of hyperlipidaemia and obesity in peptic ulcer patients. Dig Liver Dis. 2005;37:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Azuma T, Suto H, Ito Y, Muramatsu A, Ohtani M, Dojo M, Yamazaki Y, Kuriyama M, Kato T. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther. 2002;16 Suppl 2:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Lender N, Talley NJ, Enck P, Haag S, Zipfel S, Morrison M, Holtmann GJ. Review article: Associations between Helicobacter pylori and obesity--an ecological study. Aliment Pharmacol Ther. 2014;40:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Xu C, Yan M, Sun Y, Joo J, Wan X, Yu C, Wang Q, Shen C, Chen P, Li Y. Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter. 2014;19:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Du T, Chen X, Yu X, Tu L, Zhang C. Association between Helicobacter pylori infection and overweight or obesity in a Chinese population. J Infect Dev Ctries. 2015;9:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Chen TP, Hung HF, Chen MK, Lai HH, Hsu WF, Huang KC, Yang KC. Helicobacter Pylori Infection is Positively Associated with Metabolic Syndrome in Taiwanese Adults: a Cross-Sectional Study. Helicobacter. 2015;20:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Yamagata H, Kiyohara Y, Nakamura S, Kubo M, Tanizaki Y, Matsumoto T, Tanaka K, Kato I, Shirota T, Iida M. Impact of fasting plasma glucose levels on gastric cancer incidence in a general Japanese population: the Hisayama study. Diabetes Care. 2005;28:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection significantly increases insulin resistance in the asymptomatic Japanese population. Helicobacter. 2009;14:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13329] [Article Influence: 1110.8] [Reference Citation Analysis (0)] |
| 21. | Zhu A, Kaneshiro M, Kaunitz JD. Evaluation and treatment of iron deficiency anemia: a gastroenterological perspective. Dig Dis Sci. 2010;55:548-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Andres R, Muller DC, Sorkin JD. Long-term effects of change in body weight on all-cause mortality. A review. Ann Intern Med. 1993;119:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the Multiple Risk Factor Intervention Trial. Ann Intern Med. 1993;119:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Folsom AR, French SA, Zheng W, Baxter JE, Jeffery RW. Weight variability and mortality: the Iowa Women’s Health Study. Int J Obes Relat Metab Disord. 1996;20:704-709. [PubMed] |
| 25. | Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-M156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13384] [Cited by in RCA: 15964] [Article Influence: 665.2] [Reference Citation Analysis (1)] |
| 26. | Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2058] [Cited by in RCA: 2742] [Article Influence: 228.5] [Reference Citation Analysis (1)] |
| 27. | Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ohkusa T, Takashimizu I, Fujiki K, Suzuki S, Shimoi K, Horiuchi T, Sakurazawa T, Ariake K, Ishii K, Kumagai J. Disappearance of hyperplastic polyps in the stomach after eradication of Helicobacter pylori. A randomized, clinical trial. Ann Intern Med. 1998;129:712-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Sato Y, Yoneyama O, Azumaya M, Takeuchi M, Sasaki SY, Yokoyama J, Shioji K, Kawauchi Y, Hashimoto S, Nishigaki Y. The relationship between iron deficiency in patients with Helicobacter pylori-infected nodular gastritis and the serum prohepcidin level. Helicobacter. 2015;20:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Al-Haddad M, Ward EM, Bouras EP, Raimondo M. Hyperplastic polyps of the gastric antrum in patients with gastrointestinal blood loss. Dig Dis Sci. 2007;52:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |