Published online Jan 16, 2017. doi: 10.12998/wjcc.v5.i1.9
Peer-review started: June 24, 2016
First decision: August 11, 2016
Revised: September 3, 2016
Accepted: October 25, 2016
Article in press: October 27, 2016
Published online: January 16, 2017
Processing time: 202 Days and 12.1 Hours
Cardiac papillary fibroelastomas (CPFs) are the second most common primary cardiac tumors and the most common cardiac valvular tumors. Although they are histologically benign and usually asymptomatic, CPFs can lead to serious and life-threatening complications like myocardial infarction, stroke, pulmonary embolus, cardiac arrest etc. CPFs represent a rare entity in clinical medicine and literature regarding their management is limited. We report two cases which illustrate such complications arising from undiagnosed CPFs on the aortic valve. We further stress on the importance of identifying CPFs early so that they can be managed appropriately based on recommendations from the available literature.
Core tip: The cases illustrate serious complications that can arise from an undiagnosed cardiac papillary fibroelastoma. The importance of continued investigation with a transesophageal echo when there is high clinical suspicion is explained. Current management strategies have been outlined based on available literature.
- Citation: Yandrapalli S, Mehta B, Mondal P, Gupta T, Khattar P, Fallon J, Goldberg R, Sule S, Aronow WS. Cardiac papillary fibroelastoma: The need for a timely diagnosis. World J Clin Cases 2017; 5(1): 9-13
- URL: https://www.wjgnet.com/2307-8960/full/v5/i1/9.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i1.9
Primary cardiac tumors are rare and have a very low prevalence of based on 22 large autopsy studies[1]. Cardiac papillary fibroelastomas (CPFs) are the second common primary cardiac tumors[2]. For decades, CPFs were thought to be asymptomatic and incidental autopsy findings. However, in the past two decades they have gained importance as they have the potential to cause fatal and life-threatening complications. They represent a rare entity in clinical medicine and literature regarding their management is limited. We report two cases of CPFs presenting with cardiovascular and neurological complications and outline the importance of having a clinical suspicion for these tumors, in an effort to prevent further serious complications.
A 64-year-old man was transferred to our institution for further management of congestive heart failure, acute kidney injury and lower gastrointestinal bleeding. He initially presented to the other institution with worsening dyspnea and orthopnea. His medical history was significant for hypertension, congestive heart failure, and non-ST elevation myocardial infarction (NSTEMI). Prior coronary angiography was significant for non-obstructive coronary artery disease (CAD). At the other institution, a chest roentgenogram showed moderately large right-sided effusion. Transthoracic echocardiography (TTE) showed moderately reduced left ventricular systolic function with an ejection fraction of 35%-40% and severely reduced right ventricular systolic function. A diagnosis of acute decompensated heart failure was made and he was started on intravenous furosemide. Hospital course was complicated by acute kidney injury and lower gastrointestinal bleeding. The patient refused blood product transfusion as he was a Jehovah’s Witness. He did not improve clinically and was transferred to our institution. At the time of transfer to our institution, his hemoglobin was 6.3 g/dL and blood pressure was 90/40 mmHg. In our institution, ECG did not reveal ischemic changes, troponin I was elevated at 1.10 ng/mL, and brain natriuretic peptide level was elevated at 533 pg/mL. He was admitted to the medical intensive care unit for further management. Approximately 4 h after admission he developed severe hypotension which rapidly progressed to cardiac arrest with asystole. Despite resuscitative measures, patient expired.
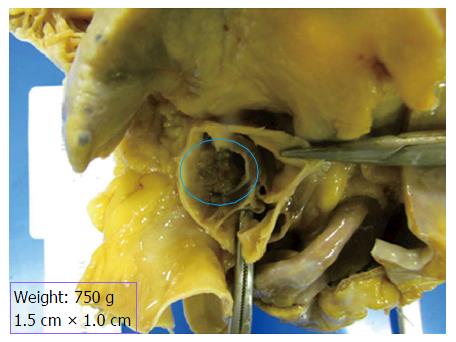
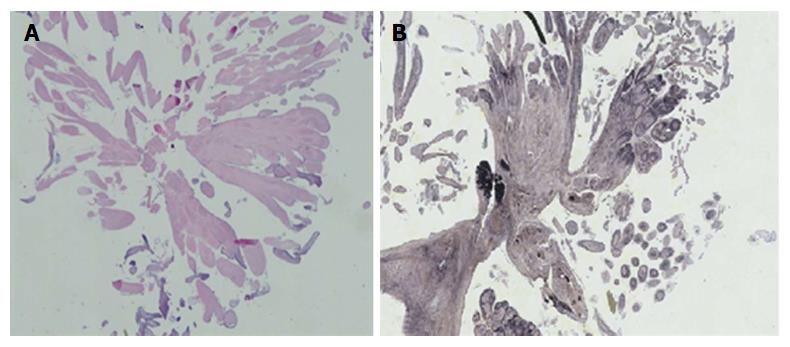
Autopsy showed cardiomegaly (750 g), old inferior wall infarct and healing acute right ventricular myocardial infarct. There was a large bulky tan-white non-encapsulated, rubbery, firm lesion with short pedicle and multiple papillary fronds on the aortic valve measuring 1.5 cm × 1.0 cm in size, completely occluding the right coronary ostium (Figure 1). Histological section of the mass shows benign papillary lesion comprised of a single layer of endocardial cells overlies a thin layer of mucopolysaccharide matrix and underlying, almost acellular, avascular stroma composed predominantly of elastic fibers. Elastin stain reveals concentric elastin fibres within the papillary excrescences (Figure 2). Immunohistochemical stains reveal lining endothelial cells are positive for S100, CD34 and Factor VIII. Coronary artery atherosclerosis of the left anterior descending artery (10%-20% occlusion), the left circumflex artery (10%-20%), and the right coronary artery (20%-30% occlusion) was seen. Coronary emboli were not noted. Based on the autopsy findings, the immediate cause of death was acute myocardial infarction secondary to papillary giant fibroelastoma of aortic valve completely occluding the right coronary ostium.
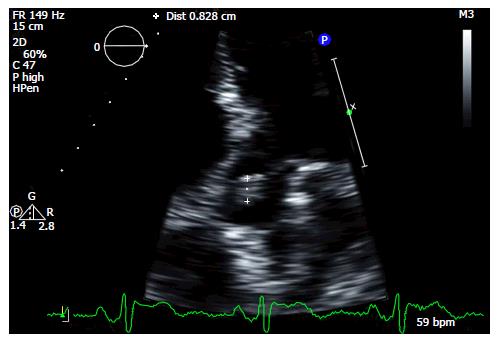
A 53-year-old female with a history of diabetes mellitus and NSTEMI presented with sudden onset tingling and numbness on her left side of her face, mouth, upper and lower extremities. Symptoms resolved spontaneously in one hour with no sequelae. Computed tomography (CT) scan of the head and CT angiography of cerebral and carotid arteries were unremarkable. Magnetic resonance imaging of the brain did not show signs of acute infarction. Based on these findings, she was diagnosed with a transient ischemic attack (TIA). Further stroke workup with a TTE showed a mobile mass on the aortic valve (Figure 3) which was later confirmed by a transesophageal echocardiography (TEE). A mobile, pedunculated, 1.2 cm round mass on the aortic side of the non-coronary cusp of a tri-leaflet aortic valve was seen in the TEE, with an appearance most consistent with a fibroelastoma. She was afebrile and blood cultures were negative, ruling out endocarditis. Attributing the TIA symptoms to embolization from the fibroelastoma, she was referred for surgical removal. Pathological exam of the resected mass confirmed a fibroelastoma. She was discharged and remained symptom free.
The first case illustrates a fatal outcome associated with an undiagnosed CPF, and the second case illustrates the benefit of early detection of a CPF to prevent further complications.
Primary cardiac tumors are very rare and have an approximate prevalence of 0.02% based on autopsy studies[1]. Myxomas were reported to be the most common, and CPFs stand second[2]. Gowda et al[3] used the term “papillary fibroelastoma” for the first time in a case report of myocardial infarction in the absence of coronary atherosclerosis.
CPFs, the most common cardiac valvular tumors, are benign endocardial papillomas, with highest prevalence in the 6th to 8th decade of life[3,4]. CPFs are predominantly valvular with 80% arising from the valvular endocardium. The aortic valve is the most commonly involved with prevalence between 35% and 63%, followed by the mitral valve (9% to 35%), the tricuspid valve (6%-15%), and the pulmonary valve (0.5%-8%)[3-5]. In a large single center review of highly-selected referral population, CPF was more prevalent (0.089% of all echocardiograms) than cardiac myxoma[4]. The pathogenesis of CPF is unclear. Papillary fibroelastomas have been described as neoplasms, hamartomas, organizing thrombi, or posttraumatic tumors[6]. Some authors believe that CPFs represent gaint form of Lambl’s excrescence, evolving from a thrombotic phenomenon due to traumatization of the endothelial cells at the level of the valves which have an increased pressure gradient[5]. Grossly, CPFs have a sea anemone appearance with a gelatinous surface and a stalk with multiple papillary projections[5]. Microscopically, CPFs are avascular and are composed of collagen, elastic, and reticulin[5].
Most patients with CPFs are asymptomatic and these are incidental findings during echocardiography, cardiac surgery, or autopsies[3]. However, the past three decades have witnessed reported cases with life-threatening cardiac and neurological symptoms, including TIA, angina, syncope, stroke, blindness, myocardial infarction and sudden death, heart failure, among many others. Cerebrovascular events are the most common presenting symptom in symptomatic patients[4]. In their meta-analysis of 725 CPF cases, Gowda et al[3] postulated that tumor mobility was the only independent predictor of CPF related death or nonfatal embolization. However, in the study by Tamin et al[4] echocardiographic characteristics of CPFs (size, location, and mobility) were not significantly associated with a cerebrovascular accident. CPFs can cause symptoms by embolization of either the tumor fragments or a thrombus that has formed on the surface of the tumor. This mechanism can explain the TIA symptoms in our second case. Direct obstruction by the tumor mass resulting in acute myocardial infarction can also occur, as in our first case. Our first patient had a NSTEMI prior to this admission with non-obstructive CAD on prior cardiac catheterization. Autopsy revealed an old inferior wall infarct and non-obstructive CAD. These findings suggest that the aortic valve CPF was causing intermittent obstruction of the right coronary ostium resulting in recurrent myocardial infarction and subsequent heart failure.
The advent of higher-resolution imaging especially TEE facilitates rapid ante-mortem diagnosis in symptomatic patients. Currently, depending on symptoms and tumor mobility either surgical resection or anticoagulation/antiplatelet agents are offered to patients. Based on available data, surgical resection is strongly recommended in symptomatic patients to prevent further cardiovascular or embolic events[3,7]. This was the rationale for surgical resection of the CPF in our second patient. She had a mobile, pedunculated fibroelastoma on her aortic valve causing a TIA (Figure 3). Curative surgical resection was done to prevent further complications. In asymptomatic patients, there is a debate between anticoagulation vs surgical excision. Increased cerebrovascular accidents and mortality were observed in patients with echocardiographically suspected CPF who did not undergo surgical removal[4]. Ikegami et al[7] recommend surgical excision in asymptomatic patients with incidental CPF finding taking into account the friability, mobility and location of the CPF. Literature suggests that successful complete resection of CPF is curative with an excellent long-term prognosis and a lower stroke risk[3,4].
Given the potential to cause fatal outcomes and the availability of successful therapies, CPF should be a diagnostic consideration in elderly patients with myocardial infarction with normal coronaries or non-obstructive CAD after other potential causes of myocardial injury and other confounding diagnoses have been ruled out. Diagnostic evaluation begins with a TTE, but it is advisable to further evaluate with TEE when clinical suspicion for CPF is high, as TTE can miss a third of CPF’s evident on a TEE[4]. It must be noted that the TTE of our first patient did not reveal a CPF. Surgical resection should be offered for symptomatic patients and also to asymptomatic patients with high risk CPFs. Close monitoring and follow-up is recommended if medical management is pursued. Randomized studies are needed to make valid recommendations as most of the available studies are single center experiences.
Case 1: A 64-year-old man with sudden cardiac arrest; Case 2: A 53-year-old female with sudden onset tingling and numbness on her left side of her face, mouth, upper and lower extremities.
Case 1: Acute myocardial infarction leading to sudden cardiac arrest; Case 2: Acute cerebrovascular event/transient ischemic attack (TIA).
Case 1: Coronary thromboembolism from atherosclerosis, acute blood loss anemia causing cardiac demand ischemia, coronary occlusion secondary to vasculitis/arteritis, aortic dissection, with retrograde involvement of the coronary arteries, coronary artery emboli, secondary to cholesterol, air, or the products of sepsis, drugs (cocaine, etc.); Case 2: Endocarditis, cardiac myxoma, cardiac papillary fibroelastomas (CPFs), organized thrombus, valvular lipoma, etc.
Case 1: Acute myocardial infarction; Case: All labs were within normal limits.
Case 2: Transthoracic echocardiography (TTE) showing a cardiac papillary fibroelastoma on the aortic valve.
CPF.
Case 2: Complete surgical excision of the CPF.
Traditionally though to be asymptomatic, the past three decades have witnessed reported cases of CPFs with life-threatening cardiac and neurological symptoms, including TIA, angina, syncope, stroke, blindness, myocardial infarction and sudden death, heart failure, among many others.
CPFs are benign endocardial papillomas and are the most common cardiac valvular tumors. They have the propensity to cause life-threatening cardiac and neurologic complications. Surgical resection should be considered in symptomatic patients and in asymptomatic patients with high risk feautures.
TTE can miss a third of the CPFs. A transesophageal echocardiography should be considered when there is high clinical suspicion. Early detection is necessary to prevent life-threatening cardiac and neurologic complications. Surgical resection can be curative.
The paper is well written.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Kataoka H, Lee TM, Peteiro J, Petix NR S- Editor: Qiu S L- Editor: A E- Editor: Li D
| 1. | Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77:107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 533] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Steger CM, Hager T, Ruttmann E. Primary cardiac tumours: a single-center 41-year experience. ISRN Cardiol. 2012;2012:906109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 471] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, Oh JK, Pellikka PA, Klarich KW. Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas. J Am Coll Cardiol. 2015;65:2420-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 5. | Darvishian F, Farmer P. Papillary fibroelastoma of the heart: report of two cases and review of the literature. Ann Clin Lab Sci. 2001;31:291-296. [PubMed] |
| 6. | Abu Saleh WK, Al Jabbari O, Ramlawi B, Reardon MJ. Cardiac Papillary Fibroelastoma: Single-Institution Experience with 14 Surgical Patients. Tex Heart Inst J. 2016;43:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Ikegami H, Andrei AC, Li Z, McCarthy PM, Malaisrie SC. Papillary fibroelastoma of the aortic valve: analysis of 21 cases, including a presentation with cardiac arrest. Tex Heart Inst J. 2015;42:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |