Published online Sep 16, 2016. doi: 10.12998/wjcc.v4.i9.269
Peer-review started: February 26, 2016
First decision: April 15, 2016
Revised: May 17, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: September 16, 2016
Processing time: 194 Days and 8.8 Hours
Hydatid cysts are a zoonotic disease that can involve many organs and tissues in the human body but primarily involve the liver and lungs. Of the main organs, adrenal glands are those seldom affected by hydatid cysts. The purpose of this study was to present a case with an incidentally detected hydatid cyst of the right adrenal gland on computed tomography, and a positive echincoccus IgG enzyme-linked immunosorbent assay test on top of a toxic multinodular thyroid goiter for which thyroidectomy was indicated.
Core tip: With an incidence of about 0.5%, Adrenal hydatid cyst disease is rarely seen, even in geographical areas where the disease is endemic, commonly occurring in the body as part of disseminated hydatid disease. Herein, we present an incidentally detected case of hydatid cyst in the right adrenal gland in a patient with hyperthyroidism secondary to toxic multinodular goiter.
- Citation: Akbulut S. Incidentally detected hydatid cyst of the adrenal gland: A case report. World J Clinical Cases 2016; 4(9): 269-272
- URL: https://www.wjgnet.com/2307-8960/full/v4/i9/269.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i9.269
Hydatid cysts are a zoonotic disease caused by echinococcal parasites from the taeniidae family[1-4]. In humans, four different types of echinococci have been identified as causing hydatid disease; however, the most frequently presented types are E. granulosus, which causes cystic echinococcosis and E. multilocularis, which causes alveolar echinococcosis[1,5-7]. E. granulosus accounts for 95% of all echinococcal diseases that occur in humans[1]. Hydatid cysts can involve almost all the organs and tissues; however, in the human body, the liver (45%-77%) and the lungs (10%-50%) are the two organs that are most frequently involved[2,4,7,8]. Even in regions where the disease is endemic, hydatid cyst disease is rarely seen in the adrenal glands. Hydatid cysts may occur in the adrenal glands as a primary (isolated) or secondary (associated with the other organs) disease. Cysts may remain asymptomatic for years, or they may be detected incidentally, during the course of radiological examinations conducted for other reasons[3]. Depending on the size, adrenal hydatid cysts can sometimes lead to complications. In this study, we present an incidentally detected case of hydatid cyst in the right adrenal gland.
A 64-year-old male patient was admitted to a private hospital with complaints of palpitation, sweating, weight loss, nausea and non-specific abdominal pain. The examination revealed a mass in the right adrenal gland, and hyperthyroidism secondary to toxic multinodular goiter. The patient’s medical history revealed no systemic diseases other than the hypertension regulated by medication. Tachycardia, tremor and mild exophthalmos were established during his physical examination. The patient’s arterial blood pressure and pulse were 150/90 mmHg and 100/min, respectively. An examination for pheochromocytoma, carried out at the other center, had not revealed any pathological result. Blood analysis at our clinic indicated the following: TSH 0.005 (0.27-4.2 μIU/mL), fT4 0.68 (0.93-1.7 ng/dL), fT3 2.36 (2-4.4 pg/mL), Na 145 (136-145 mmol/L), and K 4.7 (3.5-5.5 mmol/L). Thyroid ultrasonography revealed multiple heterogeneous nodules with solid-cystic components, the largest of which was in the left lobe-isthmus intersection (25 mm × 18 mm). Hyperactive nodular lesions were detected in the thyroid scintigraphy undertaken using 3 mCi Tc-99m pertechnetate.
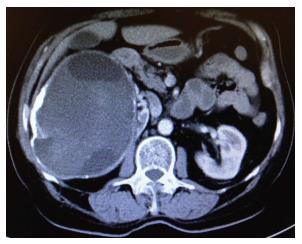
Serological analysis of the blood was reported to be Echinococcus IgG (+++). Abdominal ultrasonography revealed a lesion of heterogeneous hypoechoic solid mass of 170 mm × 120 mm originating from the right kidney, which included cystic areas. For advanced investigation, the patient initially underwent contrast-enhanced abdominal computed tomography (CT). CT revealed a 150 mm × 120 mm mass with peripheral calcification associated with multicentric hypodense cystic necrotic areas, originating from the right adrenal gland and which pressed minimally on the vena cava inferior, gallbladder and choledochus (hydatid cyst? RCC?) (Figure 1). In order to exclude malignancy potential of the mass, the patient underwent positron emission tomography/CT (PET/CT). In images obtained following an injection of 6.8 mCi F18-fluorodeoxyglucose (FDG), trapping of the FDG was not seen in the 142 mm × 121 mm mass with a calcified wall and multilocular solid-cystic components extending exophytically from the upper pole of the right kidney. Radiological signs and laboratory results indicated that the lesion in adrenal gland was in accordance with a hydatid cyst. Prior to controlling the clinical manifestations of hyperthyroidism, the patient underwent treatment with propylthiouracil (3 × 100 mg) and propranolol (1 × 50 mg). To maintain the euthyroid state, the patient initially underwent a total thyroidectomy, which was followed by daily Levothyroxine therapy of 100 mg. Based on positive echinococcus IgG enzyme-linked immunosorbent assay (ELISA) serology the patient was treated with albendazole (2 × 400 mg/d) therapy for two weeks. After prophylactic albendazole therapy, patient underwent laparotomy with right subcostal incision. No cystic lesion was seen during exploration of the liver. Surgical sponges, impregnated with 3% NaCL, were positioned in the operating site and 20 cc of 3% NaCL was injected into the cyst for about 10 min. Following this, a partial cystectomy was applied so that only the posterior wall of the cyst remained in its localization. Postoperative albendazole therapy was administered for six weeks, beginning on the post-operative 2nd day. During the post-operative 24th month control period, the disease was not seen to recur.
Hydatid cyst disease is a serious problem for public health in many parts of the world where sheep and cattle farming is the main livelihood[1,2]. Turkey is geographically equidistant between the Middle East and Mediterranean countries where hydatid cyst disease is endemic[1,2]. Although humans have no active role in the biological cycle of the disease, they can become infected accidentally by swallowing echinococcus eggs found in dog feces. After swallowing, the eggs are degraded in the digestive system and larvae are freed, which then pass through the hepatic filtration system and reach the lungs[1]. Larvae that are not trapped by the lungs’ filtration system can be established in other organs of the body such as the spleen, kidneys, brain, heart, bones, muscle tissue, pancreas, breasts, retroperitoneum, thyroid and adrenal glands, via the arterial circulation[1,5]. Sometimes larvae may directly enter the systemic circulation via the lymphatic vessels, without entering the portal vein. Rarely, they can also enter the surrounding tissue by adjacent contact[1].
With an incidence of about 0.5%, adrenal hydatid cyst (AHC) disease is rarely seen, even in geographical areas where the disease is endemic[3,4], commonly occurring in the body as part of disseminated hydatid disease. In other words, primary (isolated) AHC is an extremely rare disease[8,9]. AHCs are generally asymptomatic lesions, and are commonly detected incidentally, during radiological examinations conducted for other reasons. AHCs are rarely complicated and become symptomatic most due to symptoms developing as a result of pressure. The most frequent symptoms are pain existing due to inflammation in the adjacent tissues caused by the cyst, and those connected with the gastrointestinal system, such as distension, fullness, nausea, vomiting, constipation and loss of appetite[4]. The most severe complication of AHC disease is the rupture of cyst, causing anaphylaxis and bleeding[3]. AHC may press on the renal artery externally, but this depends on its size and localization. This condition is called Goldblatt phenomenon, and may lead to arterial hypertension[3,4,7,8]. An AHC may press on the adrenal medulla, causing pheochromocytoma-like symptoms, such as headache, palpitation and hypertension[10,11]. Another severe complication is the fistulization of AHC to the adjacent intestinal structures[5].
Because adrenal cystic lesions are extremely rare, and most of them are detected incidentally[4,6], the real incidence of it is not known, but reports suggest 0.06% to 0.18% in some autopsy series with about 92% of these lesions being unilateral[4,6,7]. With regard to morphology and etiology, these cystic lesions are separated into two groups, non-neoplastic and neoplastic. Adrenal cortical adenomas, cortical carcinomas and pheochromocytomas, may occur as cystic lesions of benign appearance[4,8]. Endothelial cysts, pseudocysts, epithelial, and parasitic cysts (6%-7%), are the most commonly occurring non-neoplastic cystic lesions[3,4,8]. AHCs account for most of the parasitic cysts. Diagnosis of AHC disease can be made by visualization methods, such as US, CT and MRI, and also by serological tests such as indirect hemagglutination test (IHA) and enzyme-linked immunosorbent assay (IgG, IgE)[6]. In the recent years, 18F-FDG PET/CT analyses are also used to exclude malignancy in adrenal lesions, as we’ve also applied in the case presented in this study.
There are only a limited number of studies in the literature relating to AHC disease, a great many of which are case reports. Therefore we have quite limited experience about the treatment of AHC disease. According to our experience, the size and localization of the cyst together with the presence of complications are the most important parameters that must be taken into consideration as the main approach to establishing hydatid cyst disease.
The most frequently applied classical method of treatment for AHC disease is the partial or total extraction of the cystic lesion using the open surgical approach, and the application of medical treatment (albendazole, mebendazole, praziquantel) in the preoperative (2-4 wk) and post-operative (4-6 wk) periods. The medical treatment aims to lower cystic pressure, to reduce the viability of cystic components, to decrease the existence of anaphylaxis, and to prevent the development of post-operative recurrence. In keeping with technological improvements, in the cases of uncomplicated AHC disease, the current tendency is to apply this method of treatment using transperitoneal or retroperitoneal laparoscopic techniques[7]. PAIR (Puncture, Aspiration, Injection and Respiration) is commonly and successfully applied in treating WHO classification stages CE1 and CE3a cysts, and in the treatment of CE1 and CE3a cysts that have recurred following medical intervention[2,4]. To the best of our knowledge, there is no study in the literature about the use of PAIR in treating AHC disease, except for the case report of Akhan et al[4]; however, in most of the studies related to AHC, it has been claimed that the PAIR method is a technique that should be avoided[10]. In our opinion, the most important reason to avoid the use of PAIR in AHC disease is leakage of cyst components from the defect on the cyst wall made by the catheter into the internal environment, with the risk of an anaphylactic reaction. However, with cysts on the parenchymal organs, such as liver, kidneys and spleen, the risk of leakage of the cyst components to the environment is fairly low, since the catheter passes through the parenchyma. We suggest the other reason is the possibility of the cystic lesion being a pheochromocytoma[4,8]. Nevertheless, we propose that it may be applied by the experienced radiologists in selected cases that have been proven, by preoperative analyses not to be malign, and in association with prophylactic albendazole therapy. Akhan et al[4], who are extremely experienced in PAIR, have also reported that PAIR could obtain successful outcomes in the cases of AHC disease when the main principles are taken into account. Another approach to AHC disease is the principle of “watch and wait”. It has been claimed that the “watch and wait” principle could be applied in the WHO Stages VCE4 and CE5 AHCs, or in cases where no complications have developed.
In the present study, we decided to apply surgical treatment, since the size of the cyst was > 10 cm, it was located in the adrenal gland, which is principally responsible for hormone synthesis, and the application of PAIR was inappropriate. An open surgical approach was also preferred due to a lack of experience regarding laparoscopic adrenal surgery. Whichever surgical treatment is applied, in a benign disease such as hydatid cyst, a resection of the cyst by protecting the adrenal gland should be attempted whenever possible[3]. Some authors claim that AHCs commonly grow to enormous sizes, leading to the destruction of the adrenal gland, and they therefore recommend a resection of the adrenal gland together with the cyst[5]. Contrary to this, some authors recommend an adrenalectomy but only in the cases where the destruction of the adrenal gland is certain[7].
As a conclusion, hydatid disease rarely occurs in the adrenal glands. Almost all of the papers related with AHC disease are case reports. When a cystic lesion is detected in the adrenal glands, hydatid cyst disease should be considered in differential diagnosis, particularly in patients living in endemic areas. Treatment should be decided by taking into consideration the parameters, such as the size of the cyst, and whether or not it is complicated. If a surgical approach is planned, we suggest that to protect the adrenal gland the cystic components should be drained and, if possible, the cyst wall should be completely resected.
A 64-year-old male patient was admitted to a private hospital with complaints of palpitation, sweating, weight loss, nausea and non-specific abdominal pain.
Hyperthyroidism, adrenal hydatid cyst disease.
Neoplastic and non-neoplastic adrenal cystic lesions, renal cell carcinoma.
Biochemical blood analysis revealed hyperthyroidism and echinococcus IgG positivity.
Contrast-enhanced abdominal computed tomography revealed a mass (150 mm × 120 mm) with peripheral calcification associated with multicentric hypodense cystic necrotic areas, originating from the right adrenal gland. 18F-fluorodeoxyglucose positron emission tomography/computed tomography revealed that a multilocular solid-cystic components (142 mm × 121 mm) extending exophytically from the upper pole of the right kidney.
Hydatid cyst disease.
Partial cystectomy + albendazole.
Hydatid cyst disease is a serious problem for public health in many parts of the world where sheep and cattle farming is the main livelihood. Cystic lesions occur more frequently in the liver, followed by the lungs. This entity is rarely seen in the adrenal glands.
AHC: Adrenal hydatid cyst; CT: Computed tomography; PET/CT: Positron emission tomography/computed tomography hybrid imaging system.
When a cystic lesion is detected in the adrenal glands, hydatid cyst disease should be considered in differential diagnosis, particularly in patients living in endemic areas.
It is a nice work except for a few missing. In this picture there is a huge cyst inferior of liver and gallbladder.
Manuscript source: Invited manuscript
Specialty type: Medicine
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akoh JA, Taheri S, Yavuz A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Akbulut S, Yavuz R, Sogutcu N, Kaya B, Hatipoglu S, Senol A, Demircan F. Hydatid cyst of the pancreas: Report of an undiagnosed case of pancreatic hydatid cyst and brief literature review. World J Gastrointest Surg. 2014;6:190-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Sozuer E, Akyuz M, Akbulut S. Open surgery for hepatic hydatid disease. Int Surg. 2014;99:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Nardi W, Buero A, Lozano S, Porto EA. Laparoscopic resection of a bulky primary adrenal hydatid cyst. J Minim Access Surg. 2015;11:279-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Akhan O, Canyigit M, Kaya D, Koksal A, Akgoz A, Yucesoy C, Akinci D. Long-term follow-up of the percutaneous treatment of hydatid cyst in the adrenal gland: a case report and review of the literature. Cardiovasc Intervent Radiol. 2011;34 Suppl 2:S256-S259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ruiz-Rabelo JF, Gomez-Alvarez M, Sanchez-Rodriguez J, Rufian Peña S. Complications of extrahepatic echinococcosis: fistulization of an adrenal hydatid cyst into the intestine. World J Gastroenterol. 2008;14:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Uludokumaci A, Canberk S, Elcin BB, Uygun N, Cakalir C, Canberk M. Hydatid disease limited to bilateral adrenal glands mimicking tuberculosis. Cytojournal. 2014;11:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kumar S, Nanjappa B, Gowda KK. Laparoscopic management of a hydatid cyst of the adrenal gland. Korean J Urol. 2014;55:493-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Geramizadeh B, Maghbou M, Ziyaian B. Primary hydatid cyst of the adrenal gland: a case report and review of the literature. Iran Red Crescent Med J. 2011;13:346-347. [PubMed] |
| 9. | Babinska A, Peksa R, Swiątkowska-Stodulska R, Sworczak K. The collection of five interesting cases of adrenal tumors from one medical center. World J Surg Oncol. 2014;12:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Tazi F, Ahsaini M, Khalouk A, Mellas S, Stuurman-Wieringa RE, Elfassi MJ, Farih MH. Giant primary adrenal hydatid cyst presenting with arterial hypertension: a case report and review of the literature. J Med Case Rep. 2012;6:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Mokhtari M, ZeraatianNejadDavani S. Primary adrenal hydatid cyst presenting with arterial hypertension. Arch Iran Med. 2012;15:328-330. [PubMed] |