Published online Aug 16, 2016. doi: 10.12998/wjcc.v4.i8.233
Peer-review started: February 23, 2016
First decision: March 25, 2016
Revised: April 15, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: August 16, 2016
Processing time: 172 Days and 20.2 Hours
Thoracic endovascular aortic repair for thoracic aortic aneurysms is an accepted alternative to open surgery, especially in patients with significant comorbidities. The procedure itself has a low risk of complications and fistulas to surrounding organs are rarely reported. An 86-year-old patient was admitted to our hospital with gastro intestinal (GI) bleeding and a suspected aortoesophageal fistula. Eight months prior, the patient had undergone a stent graft repair of a mycotic thoracic aneurysm. Computerized tomography angiography and upper GI endoscopy confirmed an aortoesophageal fistula, which was treated by esophageal stenting. With early recognition, esophageal stenting may have a role in the initial emergency control of bleeding from and palliation of aortoesophageal fistula.
Core tip: Thoracic endovascular aortic repair (TEVAR) for thoracic aortic aneurysms is an accepted alternative to open surgery, especially in patients with significant comorbidities. The procedure itself has a low risk of complications and fistulas to surrounding organs are rarely reported. Aortoesophageal fistula post-TEVAR is a very rare entity; however, it is a devastating and usually fatal condition. Treatment options are very restricted, as these patients are often not candidates for complex surgery. We consider the placement of a covered self-expanding esophageal stent to be useful in the management of secondary aortoesophageal fistula after TEVAR to prevent re-bleeding in the fragile patient.
- Citation: Tao M, Shlomovitz E, Darling G, Roche-Nagle G. Secondary aorto-esophageal fistula after thoracic aortic aneurysm endovascular repair treated by covered esophageal stenting. World J Clin Cases 2016; 4(8): 233-237
- URL: https://www.wjgnet.com/2307-8960/full/v4/i8/233.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i8.233
Thoracic endovascular aortic repair (TEVAR) is a minimally invasive and generally excellent modality to treat descending thoracic aortic aneurysms. Nevertheless, several complications including paraplegia, stroke and occasionally aortoesophageal fistula (AEF) may occur[1-4]. Aortoesophageal fistula post-TEVAR is a rare entity with a reported incidence of 1.7%-1.9%[1-3], however, it is a devastating and usually fatal condition. Treatment options are very restricted, as these patients are often not fit for complex surgery. Conservative management outcomes are almost always fatal due to recurrent hemorrhage or chronic infection and mediastinitis. In this paper, we present a case of secondary AEF post-TEVAR with insertion of a covered self-expanding esophageal stent to control gastrointestinal bleeding.
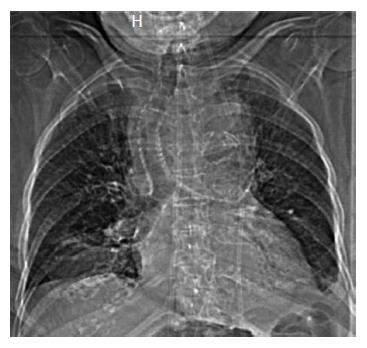
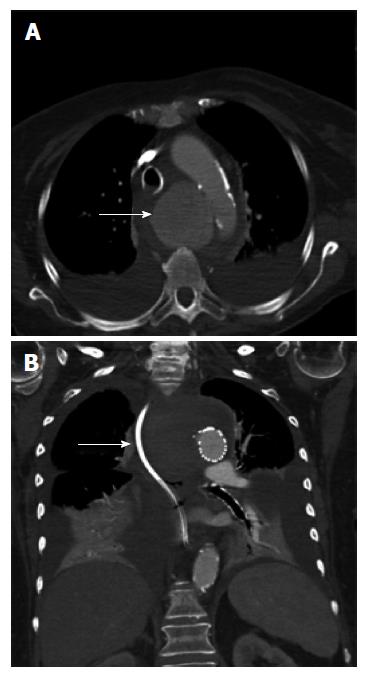
An 86-year-old woman was admitted to hospital with back pain and investigations demonstrated lumbar spine discitis. She developed progressive dyspnea, dysphagia, bilateral swelling of the arms and a leukocytosis. Chest radiography showed a widened mediastinum, bilateral pleural effusions and tracheal deviation (Figure 1). Computed tomography of the chest with intravenous contrast showed a saccular thoracic aortic aneurysm (5.0 cm × 5.0 cm) with surrounding hematoma compressing the trachea, esophagus and superior vena cava (Figure 2A). She was transferred to our center and received an endovascular stent graft (Figure 2B).
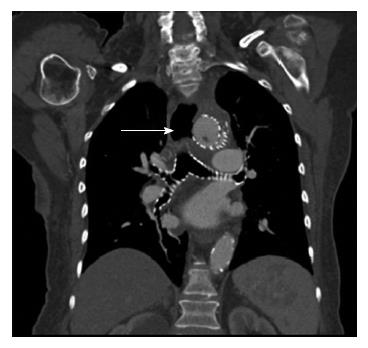
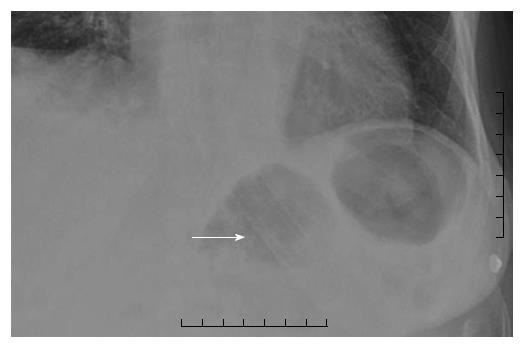
She re-presented to hospital 8 mo later following an upper gastro intestinal (GI) bleed and computerized tomography (CT) suggested an aortoesophageal fistula (Figure 3). The patient was not a candidate for open surgery and transferred for emergency gastroscopy and esophageal stent insertion. There was a large clot in the upper mid esophagus and an 18 mm diameter × 150 mm Niti-S esophageal stent (Taewoong Medical, Seoul, South Korea) was inserted to tamponade the bleeding point. The patient had persistent nausea, reflux and vomiting for 24 h post stent insertion. Chest X-ray demonstrated the stent had migrated distally resulting in these symptoms (Figure 4). She returned for repeat gastroscopy which confirmed migration of the stent through the gastro-esophageal junction. The initial stent was repositioned into the upper esophagus and a Double Niti-S™ esophageal stent (Taewoong Medical) 20 mm × 100 mm was placed, partially overlapping with the previous stent. She was commenced on lifelong antibiotic therapy and was discharged well from hospital 7 d after presentation. Unfortunately she died from a myocardial infarction 8 mo later.
The first report of aortoesophageal fistula was in 1818 due to a beef bone fragment[5]. A large review of 500 cases identified three major causes[6]. The main aetiologic factor identified being aortic disease with 54.2% of cases secondary to rupture of a descending thoracic aorta aneurysm into the oesophagus. Foreign body ingestion (19.2%) and advanced esophageal carcinoma (17.0%) were the next commonest causes.
Endovascular stent graft implantation procedures are performed in patients with aneurysms of the aorta or other large vessels. The goal of the procedure is to preserve vessel patency and to prevent the aneurysm from rupture. Formation of aortoesophageal and aortobronchial fistulas is a rare (0.5%-1.7%), but serious complication of stent graft implantation to thoracic aorta[3]. They are most frequently caused by infection of the prosthesis, compression, ischemia, local inflammatory reaction and subsequent necrosis[2]. Aortoesophageal fistulas are more common (68%) than aortobronchial (5%) and both types of fistulas coexist in 26% of cases.
Eggebrecht et al[2] followed up 268 patients undergoing TEVAR, AEF occurred in 5 (1.9%). Secondary AEF can develop late after thoracic aortic surgery in up to 1.7% of patients[7]. The incidence of post-TEVAR AEF is comparable to the incidence after open repair surgery[1]. The largest risk factor for fistula formation is infection of the prosthesis. The incidence of peri-graft infection is reported to be as high as 0.5%-5%[2]. Other factors increasing the risk of complications such as fistulas include pseudoaneurysms as in our case, emergency surgeries and intraoperative complications.
The primary presentation is massive hematemesis, often resulting in exsanguination[2]. Other presentations include: (1) severe backache[8]; (2) fever[8]; and (3) chest pain[9]. The “classic” symptom of AEF involves Chiari’s triad of aortoesophageal syndrome-chest pain, episode of small hematemesis followed by massive hematemesis[10].
The exact pathophysiologic mechanisms of AEF formation secondary to TEVAR are unknown thus far. There are different theories. A pathophysiologic mechanism of secondary AEF after TEVAR has been hypothesized[11]. Direct erosion of the rigid stent-graft through the aorta into the esophagus or pressure necrosis of the esophageal wall due to the ongoing forces of the self-expanding stent graft. Potential ischemic esophageal necrosis due to stent-graft occlusion of aortic side branches that feed the esophagus or most likely in our case infection of stent-graft prosthesis that eventually extends to the esophagus eroding its wall. Pressure necrosis mechanisms also occur in the presence of endoleak that enlarge the aneurysm sac or in an already large aneurysm sac prior to TEVAR[2,11,12].
Any patient with a previous history of thoracic aortic surgery and hematemesis should have a suspected AEF. Computed tomography angiography (CTA) is the initial investigation of choice in a case of suspected AEF looking for close approximation of the TEVAR and esophagus with or without air present. It can be confirmed by prompt upper GI endoscopy.
A treatment consensus for AEF, especially after previous TEVAR, has not been agreed upon. Conservative management of this condition almost invariably results in a fatal outcome. However, a more aggressive surgical strategy involving esophageal diversion or resection, extensive debridement, and aortic graft interposition is associated with significant mortality and morbidity[13,14]. The majority of patients who undergo TEVAR for aortic pathology have significant comorbidities precluding open surgical rescue. In this frail population management includes the use of broad-spectrum antibiotics, proton pump inhibition, and potential enteral feeding via percutaneous endoscopic gastrostomy (PEG) to bypass the esophageal fistula.
In our case we chose to place a self-expanding esophageal stent as a palliative measure. Esophageal diversion or resection was not possible due to her age and co-morbidities and re-stenting her aorta was not felt to be indicated. An esophageal stent may prevent re-bleeding and allow the patient to eat normally so is a good palliative option. When considering this option it is important to understand that stenting the “normal” esophagus is not always effective. Incomplete seal or stent migration requiring repositioning or replacement may be necessary as seen in our case. In a study of 187 esophageal stents insertions, 29 (17%) patients required repositioning or replacement[3]. There are a number of reports describing the use of self-expanding esophageal stents in this setting. Eggebrecht et al[2] inserted self-expanding esophageal stents in three patients with secondary AEF after endovascular stent grafting, with two patients surviving for 2-6 mo[2]. Onodera et al[4] reported a case where a covered self-expanding esophageal stent was inserted to prevent re-bleeding, and re-bleeding did not occur for 52 d post-insertion. In our case the patient did not re-bleed and died of a myocardial infarction 8 mo post stenting. Therefore, placement of a covered self-expanding esophageal stent may be useful to prevent re-bleeding for a substantial period of time.
The Taewoong Niti-S esophageal stents, such as the one utilized in this case encompass a family of fully covered esophageal stents for various indications. The stent design consists of a self-expanding, braided nitinol (nickel titanium alloy). The stents are covered with a polyurethane membrane throughout their length designed to resist tissue ingrowth. To reduce the risk of migration the “S” esophageal stents are designed with both proximal and distal flares which are 8 mm wider than the shaft. Such flares are meant to anchor the stents in position across an obstructing esophageal lesion or resist migration through the gastroesophageal junction. In instances similar with this case where no obstructing lesion exists, there remains a significant risk of migration. The DOUBLE Niti-S esophageal stent is designed to further minimize this risk of migration. This stent has the same basic characteristics as the “S” type esophageal stent, including flared ends and a polyurethane membrane internal covering. However, the design also includes an outer layer consisting of an uncovered nitinol mesh intended to more firmly embed into the surrounding tissues. This additional second layer also increases the radial force excreted by the stent, further reducing the risk of migration[15].
During endoscopy, there is direct visualization of the pathology and a guide wire is placed in the stomach under direct vision. Guide wire choice is best individualized to the patient’s overall situation for esophageal stent placement. Stents can be deployed under fluoroscopic guidance, endoscopic guidance, or a combination of the two. Ideally however, both modalities should be used and are complementary in the safe and accurate deployment of esophageal stents. Although the learning cure for placement of esophageal stents is not known a certain comfort level with both modalities is extremely useful in the management of these patients especially in the acute setting.
An aortoesophageal fistula presents a challenge for even the most experienced thoracic and vascular surgeons with a high mortality rate. We consider the placement of a covered self-expanding esophageal stent to be a valuable option for the management of secondary AEF after TEVAR and to prevent re-bleeding in the frail patient. The Taewoong Niti-S esophageal stent should be considered to reduce risk of migration. This treatment allows the patient to eat normally while providing the best palliation method in an often terminal condition.
An 86-year-old patient was admitted with gastro intestinal (GI) bleeding and a suspected aortoesophageal fistula.
GI bleeding on the background of aortic surgery should always raise suspicion of aorto-enteric fistula.
Hemorrhagic gastritis, esophageal varices, Mallory-Weiss tear, Neoplasm, Dieulafoy lesion or Aorto-enteric fistula.
The hemoglobin was reduced.
Computerized tomography angiography and upper GI endoscopy confirmed an aortoesophageal fistula.
A covered esophageal stent was inserted to tamponade the bleeding point.
Thoracic endovascular aortic repair (TEVAR) is a minimally invasive and generally excellent modality to treat descending thoracic aortic aneurysms. Aortoesophageal fistula post-TEVAR is a rare entity. There are few reports describing the use of self-expanding esophageal stents in this setting.
Aortoesophageal fistula post-TEVAR is an infrequent presentation likely caused by direct erosion of the rigid stent-graft through the aorta into the esophagus or pressure necrosis of the esophageal wall due to the ongoing forces of the self-expanding stent graft.
The authors consider the placement of a covered self-expanding esophageal stent to be useful in the management of secondary aortoesophageal fistula (AEF) after TEVAR to prevent re-bleeding in the fragile patient.
The author reported a rare case of AEF following endovascular repair of thoracic aortic aneurysm. The paper is well written.
Manuscript source: Invited manuscript
Specialty type: Medicine
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gao BL, Koletsis EN, Liu DL S- Editor: Qiu S L- Editor: A E- Editor: Zhang FF
| 1. | Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg. 2010;39:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Eggebrecht H, Mehta RH, Dechene A, Tsagakis K, Kühl H, Huptas S, Gerken G, Jakob HG, Erbel R. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv. 2009;2:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Lee SH, Song PS, Kim WS, Park KB, Choi SH. A case of stent graft infection coupled with aorto-esophageal fistula following thoracic endovascular aortic repair in a complex patient. Korean Circ J. 2012;42:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Onodera M, Inoue Y, Fujino Y, Kikuchi S, Endo S. A case of secondary aortoesophageal fistula inserted a covered self-expanding esophageal stent to control gastrointestinal bleeding. Case Rep Gastrointest Med. 2013;2013:857135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Dubrueil O. Observation sur la perforation de l’esophague et de l’aorte thoracique par une portion d’os oval: avec des réflexions. J Univ Sci Med. 1818;9:357-363. |
| 6. | Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, Evangelista A, Fattori R, Jakob H, Lönn L. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33:1558-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Gavens E, Zaidi Z, Al-Jundi W, Kumar P. Aortoesophageal fistula after endovascular aortic aneurysm repair of a mycotic thoracic aneurysm. Int J Vasc Med. 2011;2011:649592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Yavuz S, Kanko M, Ciftci E, Parlar H, Agirbas H, Berki T. Aortoesophageal fistula secondary to thoracic endovascular aortic repair of a descending aortic aneurysm rupture. Heart Surg Forum. 2011;14:E249-E251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Aziz S, McWilliams R, Rashid A, Gosney JR, Harris PL, Stables RH. Late aortic rupture due to stent margin pseudoaneurysm formation complicating endovascular stent graft repair of a thoracic aortic mycotic aneurysm. EJVES Extra. 2006;12:30-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Xi EP, Zhu J, Zhu SB, Zhang Y. Secondary aortoesophageal fistula after thoracic aortic aneurysm endovascular repair: literature review and new insights regarding the hypothesized mechanisms. Int J Clin Exp Med. 2014;7:3244-3252. [PubMed] |
| 12. | Girdauskas E, Falk V, Kuntze T, Borger MA, Schmidt A, Scheinert D, Mohr FW. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: successful approaches to a challenging clinical problem. J Thorac Cardiovasc Surg. 2008;136:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Czerny M, Zimpfer D, Fleck T, Gottardi R, Cejna M, Schoder M, Lammer J, Wolner E, Grabenwoger M, Mueller MR. Successful treatment of an aortoesophageal fistula after emergency endovascular thoracic aortic stent-graft placement. Ann Thorac Surg. 2005;80:1117-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Topel I, Stehr A, Steinbauer MG, Piso P, Schlitt HJ, Kasprzak PM. Surgical strategy in aortoesophageal fistulae: endovascular stentgrafts and in situ repair of the aorta with cryopreserved homografts. Ann Surg. 2007;246:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Kim ES, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH. Comparison of double-layered and covered Niti-S stents for palliation of malignant dysphagia. J Gastroenterol Hepatol. 2009;24:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |