Published online Aug 16, 2016. doi: 10.12998/wjcc.v4.i8.207
Peer-review started: March 18, 2016
First decision: April 18, 2016
Revised: April 28, 2016
Accepted: June 14, 2016
Article in press: June 16, 2016
Published online: August 16, 2016
Processing time: 150 Days and 14.6 Hours
AIM: To review all cases of abdominal tuberculosis (ATB) for demographic details, diagnostic work up and evidence of vitamin D deficiency.
METHODS: This was a retrospective analysis of all patients diagnosed with ATB from June 2003 to August 2013 at St George’s Hospital, London. Demographic data was available from the local tuberculosis database. Further clinical information was collected from electronic patient records, including radiology, endoscopy, microbiology, histology, biochemistry and serology. Patients were classified as either confirmed ATB [if mycobacteria tuberculosis (MTB) was cultured from abdominal site] or presumed ATB (if suggestive findings or high clinical suspicion). Subtypes of ATB were classified as tuberculosis (TB) peritonitis, luminal TB, solid organ TB or from a combination of sites.
RESULTS: There were a total of 65 cases identified in this time period, with a mean of 6.5 cases per year (range 4-9). Mean age 42 years, 49.2% females. Fifty-two point three percent were South Asian, 38.5% African. Forty-nine point two percent had gastrointestinal endoscopy, 30.8% paracentesis and 24.6% surgery in order to obtain samples. Forty-seven point seven percent were defined as confirmed ATB with positive culture of MTB from abdominal sites, the rest were treated as presumed ATB. Twenty-four point six percent had co-existing sputum culture positive for MTB, and 30.8% had an abnormal chest X-ray. Subtypes of ATB: 35.4% had TB peritonitis; 27.7% luminal TB; 3.1% solid organ TB; and 33.8% TB at a combination of abdominal sites. Thirteen point nine percent were human immunodeficiency virus positive, all with CD4 count less than 300 cells/μL. Seventy point five percent had severe vitamin D deficiency, and 25% were vitamin D deficient.
CONCLUSION: ATB mainly affects young South Asian and African patients, with difficulties in confirming diagnosis despite a range of non-invasive and invasive diagnostic tests.
Core tip: This is a single centre retrospective study of all cases of abdominal tuberculosis (ATB) from a single centre in the developed world. ATB remains a rare condition in the United Kingdom, which mainly occurs in young South Asians and African patients, and remains difficult to diagnose. When suspected, endoscopic biopsies must be taken in normal saline for microbiological assessment to help confirm the diagnosis. Chest radiology and sputum analysis should be performed as nearly a quarter had co-existent pulmonary tuberculosis. Vitamin D deficiency is common, and often severe, in ATB. Patients with human immunodeficiency virus and ATB present with low CD4 counts.
- Citation: Nayagam JS, Mullender C, Cosgrove C, Poullis A. Abdominal tuberculosis: Diagnosis and demographics, a 10-year retrospective review from a single centre. World J Clin Cases 2016; 4(8): 207-212
- URL: https://www.wjgnet.com/2307-8960/full/v4/i8/207.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i8.207
Abdominal tuberculosis (ATB) is a rare form of extra-pulmonary tuberculosis which can prove to be clinically challenging. It remains difficult to diagnose due to the non-specific presentation, variable anatomical location and lack of sensitive diagnostic tools[1]. ATB may occur anywhere within the abdomen, involving the gastrointestinal tract, visceral organs or peritoneum. There can be difficulty differentiating ATB from Crohn’s disease clinically, endoscopically and histologically[2,3]. Both diseases have a predilection for the small bowel, and cause chronic granulomatous inflammation[4]. There are significant clinical implications of incorrectly diagnosing tuberculosis (TB) and committing patients to a prolonged course of toxic chemotherapy; or missing TB with public health implications and causing life-threatening disseminated TB if immunosuppressant therapy is erroneously initiated.
Although TB rates in the United Kingdom have plateaued in recent years, there is still a high prevalence in the non-United Kingdom born population, predominantly in urban areas[5]. The number of extra-pulmonary cases, including ATB, is increasing with figures in England and Wales rising from 175 cases of gastro-intestinal TB in 1999, to 315 cases in 2006[6]. This increase is largely attributable to an increase in the number of non-United Kingdom-born cases, in whom extra-pulmonary TB is much more common. In London, 5% of all cases of TB were ATB in 2012[7].
There has been an emergence of a role for vitamin D beyond bone health, as an immune regulator[8]. Supplementation of vitamin D is now routine practice in the treatment of pulmonary TB (PTB), although clinical data is not conclusive and there may only be a clinical benefit in a subgroup of patients[9-11]. The association between inflammatory bowel disease (IBD) and vitamin D deficiency is well recognised[12] and has become of great interest. Animal models suggest that mice with vitamin D deficiency suffer from more severe colitis, potentially due to compromised mucosal barrier[13]. The role of vitamin D deficiency in ATB has yet to be investigated.
We report a retrospective study of all cases of ATB from an urban area, with a large migrant population, over a 10-year period. The objectives of this study were to: (1) describe the demographic profile of patients with ATB; (2) review the use of diagnostic modalities, both non-invasive and invasive, and the certainty of diagnosis of ATB; and (3) report vitamin D status of patients diagnosed with ATB.
We performed a retrospective review of patients treated for ATB at St George’s Hospital, London, from June 2003 to August 2013. Cases were identified from a local TB database, which encompasses all patients who have received treatment for TB at St George’s Hospital. Patients from this database were included in our study if they were reported as having TB involvement at an abdominal site. Cases were excluded if they were not investigated at St George’s Hospital, or if there was no evidence of involvement at an abdominal site.
Demographic data was available from the TB database. Further information on clinical investigations was collected from the electronic patient records: Radiology [including chest radiograph, ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), small bowel series], endoscopy, methods by which samples were obtained, microbiology, histology, vitamin D level and human immunodeficiency virus (HIV) status.
Ethnicity was defined as European, South Asian (Indian, Pakistani, Bangladeshi, Sri Lankan, any other Asian background), African, and Caribbean.
The reference range for vitamin D at our hospital is from 75-200 nmol/L. Vitamin D levels were classified as deficient (25-50 nmol/L) or severe deficiency (< 25 nmol/L). Vitamin D analysis was carried out using the immunodiagnostic systems total 25 OH vitamin D2 and D3 (25 hydroxy-vitamin D) assay according to manufacturer’s instruction.
In defining the accuracy of diagnosis of ATB we sub-grouped into: (1) confirmed ATB if mycobacterium tuberculosis (MTB) was cultured from an abdominal site; and (2) presumed ATB if there was suggestive histology, suggestive history, suggestive imaging. MTB was isolated from an extra-abdominal site, or if there was high clinical suspicion.
TB peritonitis was defined as ascites, peritoneal thickening or intra-abdominal lymph nodes; luminal TB was defined as TB from the oesophagus to the anus, including perianal disease; solid organ TB was hepatic or biliary involvement; combination of sites was when TB was isolated from one site, but imaging suggested concurrent involvement at other sites.
Due to small sub-group numbers comparative statistical analysis was not carried out.
Ethics committee approval was not required (as a retrospective analysis of a previously investigated clinical cohort) according to the United Kingdom National Research Ethics Service[14].
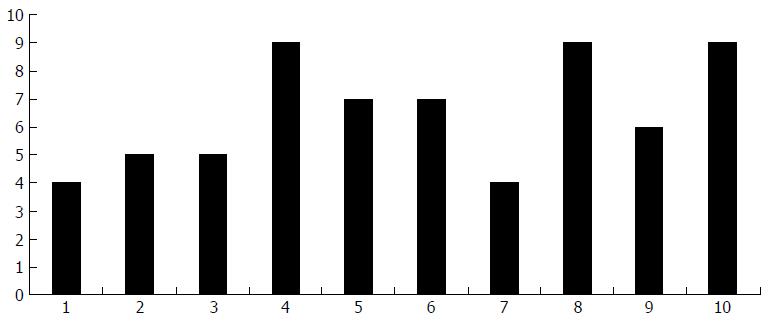
A total of 65 adults were treated at St George’s Hospital for ATB from June 2003 to August 2013. By our case definition, 31 (47.7%) were confirmed ATB, and 34 (52.3%) were presumed ATB. The number of cases of ATB over the 10-year period has remained stable, with a mean of 6.5 cases per year (range 4-9), which is demonstrated in Figure 1.
The mean age was 42 years (range 16-97 years). There were 32 females (49.2%) and 33 males (50.8%). Age and ethnicity split is shown in Table 1.
| Number | Mean age | Median age | |
| South Asian | 34 (52.3%) | 42.3 | 39.5 |
| African | 25 (38.5%) | 36.1 | 35 |
| European | 5 (7.7%) | 72.4 | 69 |
| Caribbean | 1 (1.5%) | 28 | 28 |
Sixty-four (98.5%) were screened for pulmonary TB with a chest X-ray, with 20 (30.8% of total) reported as abnormal (Table 2).
| Findings | Number of patients (n = 64) |
| Normal | 44 (68.9) |
| Consolidation | 11 (17.2) |
| Pleural effusion | 3 (4.7) |
| Lymphadenopathy (para-tracheal, hilar) | 6 (9.4) |
| Apical scarring | 1 (1.6) |
| Nodularity | 5 (7.8) |
| Focal lesion | 1 (1.6) |
| Pleural thickening | 1 (1.6) |
| Multiple radiographic features | 7 (10.9) |
| Mention of tuberculosis in differential | 6 (9.4) |
Prior to diagnosis with ATB patients underwent the following abdominal imaging modalities: 42 (64.6%) ultrasound, 46 (70.8%) CT, 2 (3.1%) MRI, and 7 (10.8%) barium small bowel series. The main findings of these are shown in Table 3.
| Ultrasound findings (n = 42) | |
| Mention of TB as differential | 3 (7.1) |
| Normal | 16 (38.1) |
| Ascites | 16 (38.1) |
| Intra-abdominal lymphadenopathy | 6 (14.3) |
| Bowel thickening | 7 (16.7) |
| Abdominal collection | 1 (2.4) |
| 2 ultrasound findings1 | 4 (9.5) |
| CT findings (n = 46) | |
| Mention of TB as differential | 22 (47.8) |
| Normal | 3 (6.5) |
| Ascites | 20 (43.5) |
| Intra-abdominal lymphadenopathy | 25 (54.3) |
| Bowel thickening | 27 (58.7) |
| Peritoneal thickening | 9 (19.6) |
| 1 CT finding2 | 17 (37.0) |
| 2 CT findings | 15 (32.6) |
| 3 or more CT findings | 11 (23.9) |
Prior to diagnosis 32 (49.2%) underwent gastrointestinal endoscopy (GIE): 18 had upper GIE; 21 lower GIE; and 7 both upper and lower GIE (Table 4).
| Upper GI endoscopy findings (n = 18) | |
| Normal | 10 (55.5) |
| Oesophageal ulcers | 3 (16.7) |
| Gastritis | 1 (5.6) |
| Gastric ulcer | 1 (5.6) |
| Duodenitis | 3 (16.7) |
| Duodenal scalloping | 1 (5.6) |
| 2 endoscopic findings1 | 1 (5.6) |
| Lower GI endoscopy findings (n = 21) | |
| Normal | 4 (19.0) |
| Colonic inflammation | 11 (52.4) |
| Ileocolonic ulcers | 5 (23.8) |
| Ileocolonic nodularity | 1 (4.8) |
| Colonic stricture | 2 (9.5) |
| Colonic polyps | 2 (9.5) |
| 2 endoscopic findings2 | 4 (19.0) |
The main invasive modality undertaken to obtain samples was endoscopy in 49.2% of subjects, paracentesis was carried out in 30.8% and surgery in 24.6%, other samples were acquired under radiological guidance, from swabs and from peritoneal dialysis fluid. The majority of specimens were sent for histology, however there was variability in numbers sent for microbiology, with endoscopic specimens being sent less frequently, and also having a lower yield (Table 5).
| n | Cytology/histology sent | Cytology/histology suggestive of tuberculosis | Microbiology sent | Culture + ve | |
| Paracentesis | 20 | 20 (100%) | 7/9 (77.8%) had lymphocytic effusion1 | 19 (95%) | 11/19 (57.9%) |
| Endoscopy | 32 | 28 (87.5%) | 14/28 (50%) | 10 (31.3%) | 3/10 (30%) |
| Surgery | 16 | 15 (93.8%) | 14/15 (93.3%) | 13 (81.3%) | 9/13 (69.2%) |
MTB was cultured from abdominal sites in 31 (47.7%) patients. Of those with culture positive ATB, 30 (96.8%) patients had fully sensitive organisms, 1 (3.2%) patient had an organism resistant to isoniazid and streptomycin.
Forty-four (67.7%) had sputum samples sent for AFB testing and TB culture, with 16 (24.6% of total) obtaining positive cultures for MTB. Of the positive MTB sputum cultures, 15 patients (93.8%) were fully sensitive and 1 (6.3%) resistant to pyrazinamide.
The site of positive cultures was 7 (11%) isolated TB from abdominal and pulmonary specimens, 24 (37%) cultured from abdominal specimens alone, 9 (14%) cultured from pulmonary specimens alone, and 25 (38%) with no positive culture.
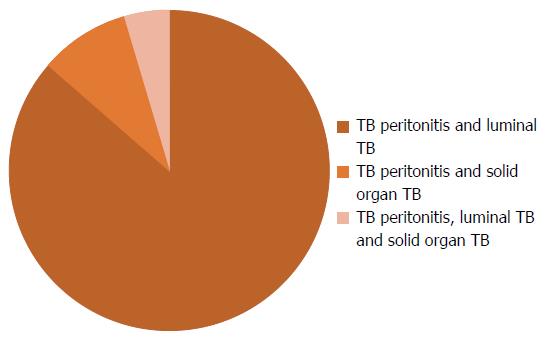
The sites are shown in Figure 2: TB peritonitis in 23 (35.4%), luminal TB in 18 (27.7%), solid organ TB in 2 (3.1%), combination of sites in 22 (33.8%).
Fifty (77%) of the patients were tested for HIV, and 9 (13.9%) were HIV positive. All HIV positive patients were non-Europeans (1 South Asian, 8 Africans). Five were diagnosed with ATB within 1 year of their HIV diagnosis. All had low CD4 counts (below 300 cells/μL), 7 (77.8%) had a CD4 count of less than 200 cells/μL, 4 had been started on antiretrovirals prior to diagnosis and had undetectable viral load.
Vitamin D levels were measured within 1 year of diagnosis (mean 1.4 mo, median 1 mo, range 0-8 mo) in 44 patients (67.7%), with a mean of 23 nmol/L (range undetectable-102 nmol/L). Of those who had measurements, 31 (70.5%) had severe deficiency (< 25 nmol/L), 11 (25%) were deficient (25-50 nmol/L).
In this study, we have reported a large modern urban case series of ATB in the developed world, where the number of cases per year has remained stable. It has mainly been detected in young minority ethnic groups, with a small group of elderly Europeans. As part of their diagnostic work up they have undergone multiple investigations, and despite this the confirmation by MTB culture occurs in less than half of cases. Nearly a quarter of patients had co-existent active pulmonary tuberculosis. Vitamin D deficiency is common and often severe in patients with ATB, and includes the whole range of demographics and phenotypes of disease.
The majority of patients were South Asian, however there was a significant cohort of African origin. Previous studies in United Kingdom investigating ATB, from Leicester[15], Blackburn[16] and Bradford[17] have shown the majority of ATB occurring in South Asians, and in London particularly in the Bangladeshi population[18]. Our series demonstrates a sizeable young African population with ATB in London, a demographic not previously reported.
Confirming a diagnosis of abdominal TB is notoriously difficult, with the rate of positive culture from abdominal sites below 50% in our series, which is similar to previously reported rates in the United Kingdom[17]. Non-invasive imaging is useful to characterise the phenotype of abdominal TB and suggest sites for sampling, however it does not assist in obtaining a definitive diagnosis, with sampling for microbiological confirmation the gold standard for diagnosis. Only 61.8% of samples acquired from invasive procedures (paracentesis, endoscopy, surgery) were sent for micobiological assessment. In our series ascitic fluid and surgically acquired biopsies had a highest diagnostic yield. There was low rate of endoscopic biopsies being sent for microbiological assessment. If TB is part of the differential diagnosis then endoscopists must ensure microbiological samples are routinely taken into normal saline solution rather than the standard formalin histopathology pots and sent for MTB culture[1]. The identification of resistant strains highlights the importance of appropriate sampling and microbiological analysis. Despite this, if ATB is suspected then empirical treatment is warranted[5], as even with extensive interventions it is not always possible to obtain positive cultures.
The clinical implications of vitamin D deficiency, and replacement in ATB, is yet to be reported. In the absence of this, data can be extrapolated from vitamin D deficiency linked with pulmonary TB[19] and inflammatory bowel disease[20], both conditions which have clinical and pathological similarities to ATB. Of particular interest is whether, as suggested in animal models, there is a compromised colonic mucosal barrier with vitamin D deficiency[13], which could exacerbate luminal and peritoneal TB in particular. Further work needs to be carried out to identify if vitamin D deficiency play a pathological role in abdominal TB and if supplementation improves treatment outcomes, however in the interim, testing and treatment of any deficiency appears to be advisable.
There are limitations to our study. The first being the retrospective design which is subject to recall bias and confounding variables, as the data set was not specifically designed for research purposes. However, our data was collected from interrogation of clinical and electronic records making this less likely and the variables studied (vitamin D, demographics and HIV) have independently been described as important factors in TB[19,21]. The second limitation is based on the definition of vitamin D deficiency which despite multiple studies has no robust classification and therefore the accepted ranges of vitamin D deficiency are variable. Also the vitamin D levels taken for our patients were not always from the time of diagnosis, and hence could be subject to other factors, such as the effect of treatment. Due to the small numbers in each sub-group (for sites, ethnicity, vitamin D levels and HIV status) comparative statistics was not possible, larger studies are needed to explore these findings further. Unfortunately, the time that different ethnic groups had been resident in the United Kingdom was unknown.
In summary, this large retrospective series reminds us that ATB is still a diagnosis to consider in individuals presenting with abdominal symptoms in the developed world, particularly in patients from ethnic minorities. Diagnosis can be challenging and requires a multidisciplinary approach with involvement from Radiology, Microbiology, Gastroenterology, Surgery, Infectious Diseases and Respiratory teams. An increase in invasive samples being sent for microbiology may assist in improving the rates of a positive diagnosis.
Abdominal tuberculosis (ATB) accounts for 5% of all cases of tuberculosis (TB) in London. There is a high prevalence of TB in non-United Kingdom born population, in previous United Kingdom based studies this has been predominantly in patients of South Asian origin. Positive confirmation of a diagnosis of ATB through culture of mycobacterium tuberculosis was achieved in 29 of 50 patients in a previous United Kingdom based series. Vitamin D replacement has been historically used in the treatment of TB, however vitamin D supplementation in the treatment of pulmonary TB (PTB) has not been shown to improve clinical outcomes.
Vitamin D has emerged as an immune regulator, with associations between vitamin D deficiency and both inflammatory bowel disease and pulmonary TB, however benefits from supplementation in PTB are not clear, and its role in ATB has not yet been identified.
This is the first United Kingdom based series which has identified patients of African origin with ATB. This is the first series of patients with ATB which has identified a prevalence of vitamin D deficiency.
This study confirms the difficulties in diagnosing patients with ATB despite a number of invasive investigations, and reinforces the importance of sending tissue for microbiological assessment. It suggests that all patients with ATB should be screened for PTB, human immunodeficiency virus and vitamin D deficiency.
This retrospective study, conducted with a large sample, is a valuable contribution to the literature on ATB. This is an interesting case series that is presented in a concise and balanced manner.
Manuscript source: Invited manuscript
Specialty type: Medicine
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kilic O, von Hahn T S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998;93:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Arnold C, Moradpour D, Blum HE. Tuberculous colitis mimicking Crohn’s disease. Am J Gastroenterol. 1998;93:2294-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kaushik SP, Bassett ML, McDonald C, Lin BP, Bokey EL. Case report: gastrointestinal tuberculosis simulating Crohn’s disease. J Gastroenterol Hepatol. 1996;11:532-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kirsch R, Pentecost M, Hall Pde M, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn’s disease and intestinal tuberculosis. J Clin Pathol. 2006;59:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Tuberculosis in the UK: 2013 report – Health Protection Agency. 2013. Available from: https://www.gov.uk/government/publications/tuberculosis-tb-in-the-uk. |
| 6. | Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax. 2009;64:1090-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Tuberculosis in London: Annual Review (2012 Data). Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/385825/TB_in_London_2012.pdf. |
| 8. | Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, Andersen PL, Glerup H, Sodemann M. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, Packe GE, Moore-Gillon JC, Darmalingam M, Davidson RN. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 397] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Chatu S, Chhaya V, Holmes R, Neild P, Kang J, Pollok RC, Poullis A. Factors associated with vitamin D deficiency in a multicultural inflammatory bowel disease cohort. Frontline Gastroenterol. 2013;4:51-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 502] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | NHS Health Research Authority. Available from: http://www.nres.nhs.uk/applications/is-your-project-research/. |
| 15. | Probert CS, Jayanthi V, Wicks AC, Carr-Locke DL, Garner P, Mayberry JF. Epidemiological study of abdominal tuberculosis among Indian migrants and the indigenous population of Leicester, 1972-1989. Gut. 1992;33:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Ramesh J, Banait GS, Ormerod LP. Abdominal tuberculosis in a district general hospital: a retrospective review of 86 cases. QJM. 2008;101:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Singhal A, Gulati A, Frizell R, Manning AP. Abdominal tuberculosis in Bradford, UK: 1992-2002. Eur J Gastroenterol Hepatol. 2005;17:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Sheldon CD, Probert CS, Cock H, King K, Rampton DS, Barnes NC, Mayberry JF. Incidence of abdominal tuberculosis in Bangladeshi migrants in east London. Tuber Lung Dis. 1993;74:12-15. [PubMed] |
| 19. | Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 449] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 20. | Ulitsky A, Ananthakrishnan AN, Naik A, Skaros S, Zadvornova Y, Binion DG, Issa M. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011;35:308-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 21. | Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 343] [Article Influence: 10.7] [Reference Citation Analysis (0)] |