INTRODUCTION
Inflammation is known to be involved in a multitude of disease processes. For instance, inflammation evokes not only inflammatory diseases such as gastroenteritis and hepatitis, but is also involved in the pathogenesis of obesity[1], diabetes mellitus[2], and neurological diseases[3]. Pro-inflammatory mediators, such as tumor necrosis factor (TNF) from fat cells causes inflammation in obesity, tumorigenesis and progression of cancer[4-7]. Recently, the mechanism of inflammation in these disease processes have been investigated and several key families of inflammatory mediators have been identified[8].
Key families of mediators include protein mediators, such as TNF and interleukins (IL)[9], and eicosanoid mediators, such as prostaglandins (PG)[10] and thromboxanes[11], which function at the cellular level[12]. Another key family of lipid mediators are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oil[13], which are signaling molecules made of lipids that regulate inflammation differently from eicosanoids and protein mediators. It has been suggested that the anti-inflammatory effects of EPA or DHA are due to their metabolite, resolvins[14,15]. Resolvins actively facilitate the resolving phase of acute inflammation, as opposed to classic protein mediators which mainly act during the onset phase of inflammation[8]. It is expected that controlling these lipid mediators will possibly prevent and/or provide treatment options for diseases related to inflammation. Herein, we review the roles of resolvins in inflammation and related diseases, and explore the potential of the lipid mediators for future clinical, therapeutic application.
AN EMERGING ROLE OF PRO-RESOLVING LIPID MEDIATORS IN INFLAMMATION
Lipid mediators are bioactive lipids produced locally by specific biosynthetic processes evoked by extracellular stimuli. In response to stimuli, they are exported to the extracellular space and bind to their specific G protein-coupled receptors on the target cells to transmit signals. They are then sequestered rapidly through specific enzymatic and non-enzymatic processes[16]. Since lipid mediators work in autocrine, paracrine, and endocrine manners, they can be regarded as local hormones or autacoids[17]. Lipid mediators regulate multiple cellular functions including cell proliferation, migration, and survival. They are related to multiple pathological conditions including autoimmune diseases, inflammatory diseases, and cancer. Lipid mediators are produced on demand, and occasionally stored as precursors to facilitate rapid mobilization when required. In the next paragraphs, the role and pathophysiology of lipid mediators in acute and chronic inflammation will be described.
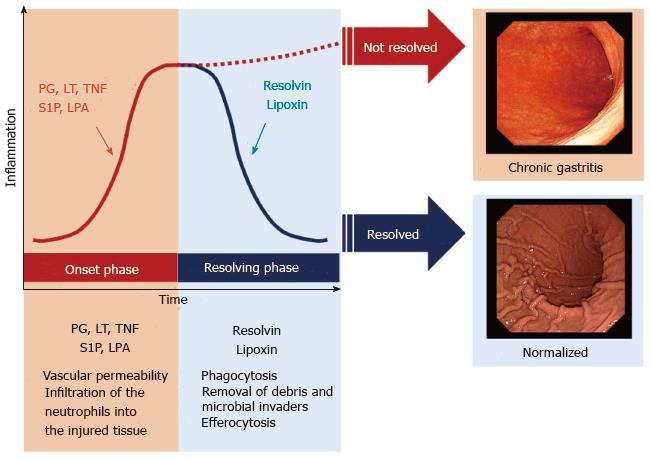
There are two phases in acute inflammation: The onset phase, and the resolving phase[8] (Figure 1). Some lipid mediators, such as eicosanoids, play important roles in both phases (Figure 2). During the onset phase, lipid mediators such as PG and leukotrienes (LT) function as pro-inflammatory mediators, in addition to cytokines such as TNF and IL. After tissue injury or trauma, PGE2 and LTB4 increase vascular permeability, initiate infiltration of the neutrophils into the injured tissue, and remove dead cells. Further, it has been revealed that sphingolipids, such as sphingosine-1-phosphate, and lysophosphatidic acid, also play a critical role as pro-inflammatory lipid mediators in the onset phase of acute inflammation[18-22]. Although the role of pro-inflammatory lipid mediators during the onset phase has been well studied, the mechanisms of pro-resolving lipid mediators in the resolving phase of acute inflammation has not been as well elucidated.
Figure 1 A course of acute inflammation with two phases: The onset phase and the resolving phase.
Acute inflammation is caused by stresses such as tissue injuries, microbial infections or surgical intervention. There are two phases in acute inflammation: The onset phase, and the resolving phase. Usually, the inflammation is resolved through these phases. When the inflammation is not resolved, it develops diseases with chronic inflammation. For instance, inflammation in stomach caused by microbial infections follow a course of the two phases in acute inflammation. When the acute inflammation is not resolved through the resolving phase, it develops chronic gastritis. PG: Prostaglandins; LT: Leukotrienes; TNF: Tumor necrosis factor; S1P: Sphingosine-1-phosphate; LPA: Lysophosphatidic acid.
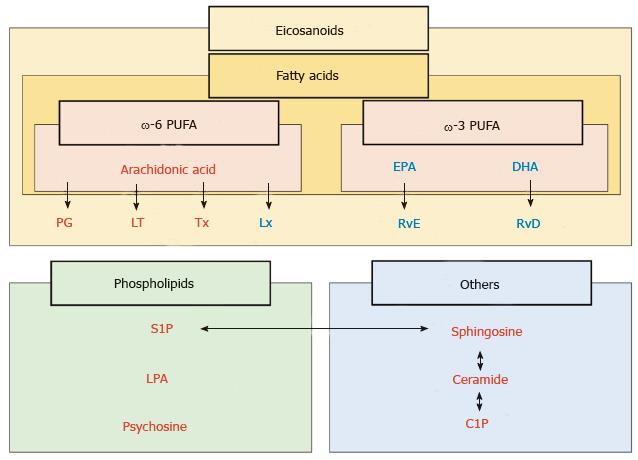
Figure 2 Classification of lipid mediators of inflammation.
Lipid mediators are classified with fatty acids, phospholipids and others. Among the fatty acids, eicosanoids are the derivatives of polyunsaturated fatty acids (PUFA), which are present in dietary sources, such as fish oil (ω-3 PUFA) and vegetable oil (ω-6 PUFA). ω-6 PUFA generates prostaglandins (PG), leukotrienes (LT), thromboxanes (Tx) and lipoxins (Lx). Resolvin originates from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are components of ω-3 PUFA. Resolvin derived from EPA and DHA are termed resolvin E (RvE) and resolvin D (RvD) series, respectively, both of which act as pro-resolving mediators. Phospholipids including sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) and others including ceramides and ceramide-1-phosphate (C1P) usually act as pro-inflammatory mediators. Red: Pro-inflammatory mediators; Blue: Pro-resolving mediators.
The classic model of resolution of acute inflammation includes sequestration of pro-inflammatory cytokines, clearance of neutrophils from epithelial surfaces, phagocytosis of apoptotic neutrophils, and removal of inflammatory debris and microbial invaders[23]. This has been traditionally considered a passive process that limits excessive infiltration of neutrophils, however, it has been recently discovered that lipid mediators actively facilitate the resolution of acute inflammation to prevent excessive inflammation[24,25]. At the height of the inflammatory response, pro-resolving lipid mediators are produced, and promote resolution of the inflammation[8]. Pro-resolving lipid mediators stimulate macrophage switching from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype in the latter part of onset phase[26]. During the transition from the onset phase toward the resolving phase, representative pro-resolving lipid mediators, such as lipoxin and resolvins, actively stimulate the recruitment of non-inflammatory monocytes. Lipoxin is a metabolite of the arachidonic acid pathway and plays a vital role in reducing excessive tissue injury and chronic inflammation. In the resolving phase, both resolvin and lipoxin play important roles in phagocytosis, removal of debris and microbial invaders, and efferocytosis (Figure 1). Efferocytosis is the process where neutrophils and dead cells presenting for apoptosis are taken into the phagocytes and removed from the site of inflammation[27]. When efferocytosis does not work well, neutrophils are not dealt with adequately and they release inflammatory cytokines. Lack of efferocytosis results in prolonged chronic inflammation (Figure 1)[28,29]. Considering that resolvins promote efferocytosis in the resolving phase, and it is reasonable to assume that the lack of resolvin results in prolonged inflammation. Indeed, it has been reported that supplementation with pro-resolving mediators reduces the development of chronic inflammation in animal studies. Taken together, it is suggested that resolvins have the clinical potential to slow the progression of chronic diseases.
HISTORY OF ω-3 POLYUNSATURATED FATTY ACID AND RESOLVINS
Increasing evidence has revealed that acute and chronic inflammation are involved in the pathogenesis of many diseases, such as vasculopathies[30], metabolic syndrome[31], neurological diseases[32], and cancer[7]. Elucidation of the mechanism of pro-resolving mediators in these diseases may provide new insights into treatment options[33-35]. Among the previously-discovered pro-resolving lipid mediators, resolvins have been shown to play a central anti-inflammatory role[36].
In the 1970s, an epidemiological study revealed the benefits of dietary ω-3 polyunsaturated fatty acids (PUFA) for the first time, whereby coronary atherosclerosis occurred far less among Greenland Eskimos, who consume more fish oil, than people in industrialized countries who do not consume as much fish oil[37]. Although ω-3 PUFA has long been thought to have anti-inflammatory properties, the molecular mechanism of how they resolve inflammation is unclear.
Dr. Serhan is credited with first discovering a group of new compounds that were generated in resolving inflammatory exudates in murine air pouches with ω-3 PUFA. He hypothesized that those new compounds play an active role in the process of resolving inflammation through ω-3 PUFA. Indeed, these new compounds proved to have the potential to inhibit human leukocyte migration and infiltration in vivo[15]. In 2000, Dr. Serhan identified a new lipid mediator derived from ω-3 PUFA that promoted the resolving phase of acute inflammation and it was later named “resolvin”[14]. Recently advances in mass spectrometry have enabled accurate quantification of the mass amounts of lipid mediators, including resolvins, and gradually have demonstrated their function.
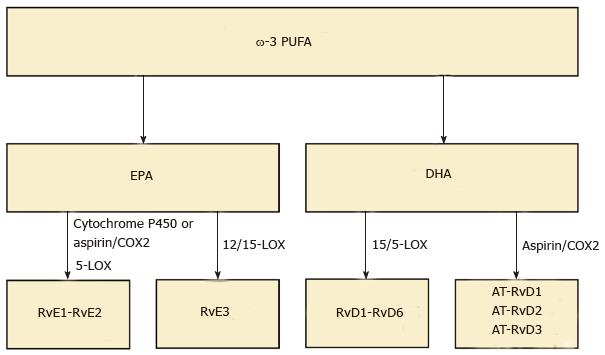
PUFA are present in various dietary sources, such as fish oil (ω-3 PUFA) and vegetable oil (ω-6 PUFA). ω-6 PUFA generates arachidonic acids which are the precursors to PG, LT and lipoxin. ω-3 PUFA generates EPA and DHA. Resolvins derived from EPA and DHA are termed resolvin E (RvE) and resolvin D (RvD) series, respectively[14] (Figure 2). Resolvins are biosynthesized from ω-3 PUFA by specific enzymes including lipoxygenase (LOX). RvD1 is biosynthesized via 15/5-lipoxygenase (LO) within vascular endothelial cells. To date, six members of this family has been identified (RvD1-6)[38]. Moreover RvD is also biosynthesized via aspirin-acetylated COX-2, and we call aspirin triggered RvD (AT-RvD). Although the structures and biosynthetic routes of RvDs are identified, their physiological functions remain to be clarified.
RvEs are endogenously produced during endothelial cell-leukocyte interaction. The complete stereochemistry revealed that the first member of this family, RvE1, has multiple biosynthetic routes. Within vascular endothelial cells, aspirin-acetylated COX-2 converts EPA into 18R-hydro (peroxy)-eicosapentaenoic acid (HEPE) through the enzymatic reaction of 5-LOX. HEPE is rapidly taken up by activated leukocytes and further metabolized into RvE1[39]. RvE1 is also produced through an aspirin-independent pathway via cytochrome P450-driven oxygenation of EPA[40]. RvE2 is also biosynthesized in endothelial cells like RvE1. RvE1 and RvE2 are mainly biosynthesized in neutrophils, and react with 5-LOX, which is known to increase during the onset phase of acute inflammation[39,41].
RvE3 is generated from 18-HEPE via the 12/15-LOX pathway. A recent study demonstrated that eosinophils contribute to the biosynthesis of RvE3. RvE3 inhibited neutrophil chemotaxis in vitro at low nanomolar concentrations. It is, therefore, intriguing to consider RvE3 as a potential endogenous anti-inflammatory compound to protect against an aberrant or uncontrolled innate inflammatory response[42] (Figure 3).
Figure 3 Classification of resolvins.
Resolvins are biosynthesized from ω-3 polyunsaturated fatty acids (PUFA) by specific enzymes including lipoxygenase (LOX). Resolvin E (RvE) is endogenously produced from eicosapentaenoic acid (EPA) during endothelial cell-leukocyte interaction. RvE1 and RvE2 are biosynthesized through two different pathways via cytochrome P450-driven oxygenation of EPA or via aspirin-acetylated cyclooxygenase 2 (COX-2). RvE3 is generated via the 12/15-LOX pathway. In addition, resolvin D (RvD) is biosynthesized from docosahexaenoic acid (DHA) via 15/5-LOX within vascular endothelial cells. To date, six members of this family has been identified RvD1-6. Also, RvD is also biosynthesized via aspirin-acetylated COX-2, and we call aspirin triggered RvD (AT-RvD).
LIPID MEDIATORS IN INFLAMMATORY DISEASE
It has been shown that excessive inflammation contributes to widely occurring human diseases. Various kinds of natural foods such as fish oil and plants contain ω-3 PUFA, and many studies have been conducted to examine its benefits on human health. Many clinical studies related to ω-3 PUFA have been conducted since the Greenland Eskimos study in 1970s, and have revealed that it improves various disease conditions, especially those with acute and chronic inflammation. Although it has been revealed that ω-3 PUFA displays anti-inflammatory effects, the underlying mechanisms were unknown. Later, it has been discovered that resolvins play a pivotal role in resolving inflammation through ω-3 PUFA. Although there have only been a small number of studies testing the effect of resolvins in humans, it remains very promising considering previous positive results with ω-3 PUFA[43-46]. Further, recent advances in mass spectrometry have enabled quantification of absolute amounts of specific lipid mediators[36]. This has enabled measuring the amount of specific resolvins in human tissues, such as blood or sputum at different time points in inflammatory conditions. These studies have revealed the importance of resolvins in the inflammatory disease processes in the human body. In the following paragraphs, representative studies describing pro-resolving lipid mediators in various human diseases will be reviewed.
Wang et al[43] reported a randomized clinical trial, in which 64 adult patients with gastrointestinal diseases, such as gastric cancer and colonic cancer, were randomly assigned to total parenteral nutrition with either ω-3 PUFA enriched emulsion or medium-chain triacylglycerols/long-chain triacylglycerols for 5 d after surgery. They found that the ω-3 PUFA enriched emulsion significantly increased the concentrations of pre-resolving mediators leukotriene B5 and B4, and significantly decreased the inflammatory cytokines IL-6, TNF-α, and nuclear factor-κB in plasma. This result suggests that ω-3 PUFA in total parenteral nutrition has an anti-inflammatory effect in patients with gastrointestinal diseases[43].
Peritonitis is defined as an inflammation of the peritoneum, commonly due to intraperitoneal bacterial or fungal infection associated with bowel perforation. Spite et al[44] demonstrated that intraperitoneal administration of RvD2 improved survival of peritonitis in mice by enhancing bacterial clearance without immune suppression in a cecal ligation and puncture-initiated sepsis model.
Tomio et al[46] investigated the effect of dietary ω-3 PUFA in inflammation by monitoring cystic endometriotic lesions in fat-1 mice, which are genetically modified to convert ω-6 PUFA into ω-3 PUFA[45]. Endometriosis is an inflammatory disease of the female reproductive tract associated with ectopic implants of endometrial tissue. The number and weight of cystic endometriotic lesions in fat-1 mice two weeks after inoculation were less than half to those of controls where all of them were fed a special diet high in ω-6 PUFA and low in ω-3 PUFA. They concluded that ω-3 PUFAs protects against the development of endometriosis[46].
Resolvins have been shown to demonstrate a protective effect in kidney diseases. Zivkovic et al[47] reported serum lipid mediator levels analyzed by mass spectrometer in IgA nephropathy patients who were supplemented with either fish oil or corn oil placebo. IgA nephropathy patients supplemented with fish oil showed lower production of arachidonic acid metabolites in serum and improved proteinuria[47]. Furthermore, Zhang et al[48] found that RvD1 is protective against Adriamycin-induced podocyte damage in a mouse model of acute kidney injury.
Acute lung injury and other lung diseases lack effective curative treatments. Cystic fibrosis (CF) is an autosomal recessive disease that causes chronic inflammation in the lung due to chronic obstructive pulmonary disease and frequent lung infections. Yang et al[49] measured RvE1 in sputum from CF patients using mass spectrometry. Patients with detectable levels of the RvE1 in sputum demonstrated better lung function compared to patients with undetectable levels, indicating that higher concentration of the RvE1 in sputum is associated with less inflammation in the lungs of CF patients[49]. Wang et al[50] found that pretreatment with RvD1 reduced the lung damage in a mouse model of acute lung injury and acute respiratory distress syndrome induced by oxidative stress of lipopolysaccharide by decreasing the recruitment of neutrophils and stimulating their apoptosis. These clinical, translational studies suggest the important role of the lipid mediators in these diseases.
The role of resolvins has been assessed not only in acute, but also in chronic inflammatory diseases. The complications of diabetes mellitus involve chronic inflammation-mediated micro- and macro-vascular damage, disruption of lipid metabolism, and abnormalities of neutrophil-mediated events. Herrera et al[51] used transgenic mice over-expressing the human RvE1 receptor in type 2 diabetes mice and investigated the impact of RvE1 on the phagocytosis of Porphyromonas gingivalis in type 2 diabetes. They found that RvE1 increased the neutrophil count and that phagocytosis of Porphyromonas gingivalis was significantly increased in mice over-expressing the human RvE1 receptor[51]. Atherosclerosis is another chronic inflammatory disease of the vascular wall. Miyahara et al[52] utilized the balloon induced arterial injury in rabbits and found that RvDs broadly reduced vascular smooth muscle cell responses to vascular injury.
Amyotrophic lateral sclerosis (ALS), Parkinson’s, Alzheimer’s, and Huntington’s disease are neurodegenerative diseases associated with progressive loss of structure and/or function of neurons. Xu et al[32] found that RvD1 inhibited the pro-inflammatory responses of inflammatory mediators induced by LPS in murine microglia. This is significant because the inhibition of microglia-activated inflammation is an accepted potential treatment strategy for these diseases[32].
There has been an interest in whether the increasing dietary intake of ω-3 PUFA may improve cognitive function. Hashimoto et al[53] divided healthy people over 55 years old in two groups; people taking DHA 1.7 g and EPA 0.4 g, and people consuming olive oil. The four-year cohort study identified a delay in deterioration of cognitive function among people in the DHA and EPA group, with higher scores in MMSE (mini-mental state examination). This indicated that taking ω-3 PUFA may slow the deterioration of cognitive function in aging[53]. However, Quinn et al[54] subsequently divided patients with Alzheimer disease into two groups; the group of patients taking DHA 2 g and those taking corn oil. After an 18 mo double-blinded follow-up, they concluded that supplementation with DHA compared with placebo did not slow the rate of cognitive and functional decline in patients with mild to moderate Alzheimer disease[54]. Though the effect of ω-3 PUFA in cognitive function remains unclear, the mechanism of ω-3 PUFA likely includes its anti-inflammatory action.
LIPID MEDIATORS IN CANCER
Cancer is one of the leading causes of death worldwide, accounting for 8.2 million deaths in 2012. Inflammation is involved with cancer progression, especially among certain types of cancer caused by viral infection[27,55]. Such examples include hepatitis C virus and liver cancer[56,57], human papillomavirus and cervical cancer of the uterus[58,59], Helicobacter pylori and gastric cancer[60,61], and schistosomiasis infection and bladder cancer[62,63]. Further, some autoimmune conditions that cause inflammation can lead to cancer, such as autoimmune hepatitis and liver cancer[64]. Chronic inflammation can also lead to cancer, such as reflux esophagitis and esophageal cancer[65,66], anal fistula and fistula cancer[67], and inflammatory bowel disease and colorectal cancer (CRC)[68].
In CRC patients, dietary ω-3 PUFA levels have been associated with reduced blood levels of IL-6 and increased albumin. Moreover, in CRC patients undergoing chemotherapy, supplementation with dietary 0.6 g/d of EPA + DHA for 9 wk reduced C-reactive protein levels, indicating less systemic inflammation. Furthermore, it has been shown that the level of total ω-3 PUFA is lower in the serum of CRC patients compared to healthy controls[69].
In breast cancer, ω-3 PUFA is receiving a lot of attention. Leslie et al[70] studied whether ω-3 PUFA reduces breast tumors in female transgenic mice. They fed mice a fish oil- containing ω-3 PUFA diet for 20 wk and measured tumor onset, size, and multiplicity. They found that tumor size significantly reduced in a dose dependent manner. In addition, in a study by Kiyabu et al[71], 38234 Japanese women aged 45-74 years were followed for a mean duration of 14.1 years and diagnosed with 556 new breast cancers. They found that development of estrogen receptor positive (ER+), progesterone receptor positive (PR+) breast cancers, which are a favorable breast cancer phenotype, were positively associated with intake of ω-6 PUFA, whereas it was negatively associated with intake of ω-3 PUFA. Overall breast cancer risk, however, was not associated with the intake of combined fish oil PUFA (ω-3 PUFA and ω-6 PUFA)[71]. This provides evidence of an interaction between fish oil PUFA and breast cancer phenotype, however additional studies are required to more clearly elucidate the associations.
Cancer is not only generated by genetic alterations as a result of intrinsic or exogenous mutagens, but also by long-term exposure to acute or chronic inflammation. Persistent inflammation might promote cell transformation through DNA damage, which is one of critical processes of carcinogenesis. Inflammation can also lead to the release of matrix metalloprotease, high mobility group box, which promotes cancer progression by regulating replicative potential, angiogenesis, apoptosis, and self-sufficiency in growth signals[72]. Considering that resolvin is a potent anti-inflammatory mediator, we cannot help but speculate that it suppress cancer progression in the context of inflammation-related carcinogenesis. In fact, Kuang et al[73] found that resolvin D1 and E1 prevent liver injury and the changes of hepatitis to liver cancer in mice. Moreover, resolvins have been shown to suppress epithelial mesenchymal transition (EMT), which is a key event in metastasis of cancer. Interestingly, Lee et al[74] found that resolvins inhibited transforming growth factor (TGF)-β1-induced EMT of A549 human lung cancer cells in vitro. TGF-β1, which is known to induce EMT of lung cancer cells, is one of the featured mediators in the resolution of inflammation and key components comprising the tumor microenvironment. Thus, modulation of TGF-β1 by resolvins offers therapeutic potential in treating and/or preventing metastatic spread of cancer[75]. Currently, there are only a small number of studies that investigate the correlation between resolvins and cancer in humans, but the spread of tandem mass spectrometry will make it possible to gain more data regarding the suppressive or protective effect of resolvins on cancer progress not only in experimental models, but also in human patients.
FUTURE DIRECTIONS
Given its unique function with minimal side-effects, resolvins could represent a new class of anti-inflammatory drugs. Currently, non-steroidal anti-inflammatory drugs (NSAIDs), steroids and opioids are commonly used to treat inflammation and pain caused by injury. NSAIDs in particular are used for a wide spectrum of pathologies including short- and long-term pain relief and various inflammatory conditions. However, NSAIDs can cause several serious adverse effects such as gastric ulceration and renal damage, which are frequently observed in patients with arthritis and other rheumatologic conditions requiring chronic NSAID use[76]. The potential side effects with steroids are well known with systemic and psychological side effects including abnormal lipogenesis and fat distribution, adrenal insufficiency, osteoporosis, glaucoma, menstrual irregularity, and immune compromise[77]. Despite these side effects, steroids remain the first-line therapies for several diseases such as inflammatory bowel disease, rheumatoid arthritis and other autoimmune conditions. We cannot help speculate that treatment with lipid mediators, such as resolvins, may be a breakthrough for these patients.
It is well established that aspirin decreases the development and progression of colon adenomas. Aspirin irreversibly inactivates the cyclooxygenase enzyme, thus decreasing the production of inflammatory precursors PG and thromboxanes. However, the side effects of aspirin have prevented it to be widely used for chemoprevention of colon cancer. Hull et al[78] are currently conducting a trial investigating whether EPA is as effective as aspirin, or has additive effect. Another ongoing trail randomizes patients with colonoscopy-identified colon adenomas to either EPA intake or placebo for one year with measured levels of ω-3 PUFA and RvE1 in plasma, urine, erythrocytes and rectal mucosa. These studies will provide further evidence regarding the efficacy of resolvins in affecting cancer progression.
It is widely accepted that preoperative optimization of immune function improves the postoperative outcome. Due to the minimal side effects, resolvins could be given perioperatively unlike many other drugs due to its absence of impact on wound healing. It is well known that surgical stress and inflammation cause global metabolic suppression. Preoperative restoration of immune function could potentially reduce postoperative infectious complication, reduce use of resources, and improve patient outcomes. It may also prompt early ambulation and avoid disuse syndrome.
CONCLUSION
In summary, ω-3-PUFA plays important roles in inflammation-related diseases, with resolvins playing a central mechanistic role. Resolvins may be a key player for treatment of inflammation-related diseases. Further investigation will be needed to develop new therapies utilizing the anti-inflammatory lipid mediators for people suffering with those inflammation-related diseases and cancers.