Published online Jun 16, 2016. doi: 10.12998/wjcc.v4.i6.151
Peer-review started: February 28, 2016
First decision: March 24, 2016
Revised: April 12, 2016
Accepted: May 7, 2016
Article in press: May 9, 2016
Published online: June 16, 2016
Processing time: 96 Days and 12.3 Hours
A rare entity of persistent mullerian duct syndrome usually presents with a common symptom of undescended testis (UDT) or hernia. Male pseudo-hermaphroditism with persistent internal mullerian duct structures can present with a 46, XY karyotype with normal external genitalia and. It arises due to deficiency of anti-mullerian substance, resulting from reduced production/responsiveness to mullerian duct, leading to persistence of mullerian duct along with normal development of Wolffian duct structures. Presence of mullerian structure prevents testicular descent increasing the risk of testicular vanishing syndrome. The authors here report a case of 16 years old phenotypical male who came with retractile right sided testis and left side UDT in the urology out-patient department. Explorative laparotomy was performed and an ill-defined mass was excised and sent for histopathological examination. Histopathology revealed presence of mullerian structures. The serum testosterone level was normal, buccal smear cytology and karyotyping revealed a 46, XY genotype of the patient.
Core tip: Undescended testis is a common cause of infertility. However persistence of mullerian structures in such a case is rare entity. This case highlights how early recognition of this entity can prevent vanishing testis syndrome and infertility.
- Citation: Vanikar AV, Nigam LA, Patel RD, Kanodia KV, Suthar KS, Thakkar UG. Persistent mullerian duct syndrome presenting as retractile testis with hypospadias: A rare entity. World J Clin Cases 2016; 4(6): 151-154
- URL: https://www.wjgnet.com/2307-8960/full/v4/i6/151.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i6.151
Persistent mullerian duct syndrome (PMDS) is recognized as a form of malformative disorder of sex development (DSD) of patients having “Y” chromosome[1,2]. PMDS is a type of male pseudo-hermaphroditism, a rare type of DSD that is characterized by presence of mullerian duct derivatives in a phenotypical male with a karyotype of 46, XY. The cause is attributed to insufficient amount of mullerian inhibiting substance-(MIS)/factor (MIF) or anti-mullerian hormone (AMH) or its insensitivity to the mullerian duct. Genetically two types of PMDS are identified, type-I, which is male type and type-II which is female type[2,3].
We report a case of type-I PMDS who presented with left sided undescended testis (UDT) and right sided retractile testis with presence of mullerian structures on histopathological examination.
A boy of 16 years old was referred at her outpatient clinic with complaint of urinary leak from dorsal penile region. He was operated for second degree hypospadias, a month back following which he developed the present complaint. Informed written consent was taken from the parents.
On general examination, the patient was conscious, oriented and well-nourished with normal secondary sexual characteristics for his age. On local genital examination, uretero-cutaneous fistula was identified with retractile right testis and left scrotal sac appeared empty. The remaining systemic examination was unremarkable. Hence diagnosis of left sided cryptorchidism was made. Ultrasound revealed abdominal location of left testis. Karyotyping revealed a normal 46, XY karyotype. Buccal smear cytology showed absence of Barr bodies. Serum total testosterone levels was 188.6 ng/dL (normal reference range: 100-200 ng/dL). Explorative laparotomy was performed and an ill-defined mass was excised from the left pelvic region. The tissue was sent for histopathological examination. Gross examination revealed irregular, grey-white tissue altogether measuring 4.5 cm × 3 cm × 1 cm in size with a cord like structure measuring 5 cm in length was also identified. Multiple sections were taken from the submitted tissue and stained with hematoxylin and eosin stain.
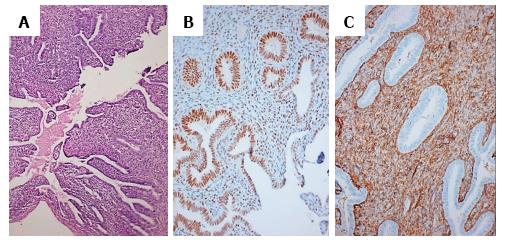
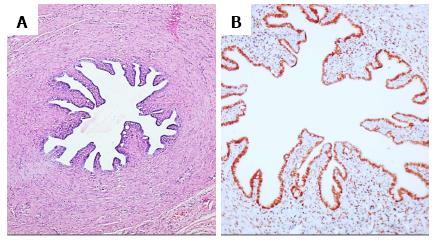
Microscopy revealed infantile uterus comprising of fibromuscular perimetrium, unremarkable myometrial tissue and endometrial tissue. The endometrium comprised of round to slightly elongated tubular glands lined by columnar to low cuboidal epithelium, surrounded by compact, deeply basophilic stroma comprising of spindly cells. Few endometrial glands showed early dilatation and tortuosity with basal nuclei and apical secretory vacuolations. These glandular lumina revealed secretions (Figure 1A). A different focus showed fallopian tube having fibromuscular wall with innermost oblique, middle circular and outermost longitudinal muscle layer, mucosal lining comprising of ciliated columnar cells, occasional secretory cells and plical folds. The stroma revealed a storiform pattern with plump spindly cells (Figure 2A). Cystic structures lined by ciliated columnar epithelium were also present which favored histology of appendix epididymis.
Immunohistochemistry for estrogen receptor (ER) and CD10 was performed. The cells of the endometrial glands (Figure 1B and C) and mucosal cells of fallopian tube (Figure 2B) along with few stromal cells revealed ER and CD10 positivity. Presence of mullerian duct structures in a patient with karyotype 46, XY confirmed the diagnosis of PMDS.
DSD are disorders related to abnormal development of sex organ in patients having Y chromosome. These are classified as malformative, dysgenic and non-dysgenic DSDs. PMDS first described by Nilson in 1939, is a type of non-dysgenic DSD characterized by presence of derivatives of mullerian duct (uterus, cervix, fallopian tubes, upper two thirds of vagina) in a male with karyotype of 46, XY. Mullerian duct structures are usually present in the male and female fetus until the eighth week of gestation following which the fetal sertoli cells start releasing MIS/MIF which leads to regression of mullerian duct in male fetus[1-3]. Overall incidence is reported as 0-1 per 100000 live births[4]. Anatomically three forms of PMDS are identified. Type-I (male type) PMDS, most common form seen in 60%-80%, type-II (transverse testicular ectopia) in 20%-30% and type-III (female type) is rare, noted in 10%-20% cases[5]. PMDS may occur sporadically, inherited as an X-linked or autosomal dominant sex-limited trait. Rarely it can be found in association with Turner’s syndrome, Mayer-Rokitansky Kuster Hauser’s syndrome or Klinefelter’s syndrome[6]. The exact pathogenic mechanism is known in nearly 85% and unknown in 15% cases. The type-I PMDS (45%) occurs usually due to deficiency of AMH whereas type-II PMDS (40%) occurs due to defects in AMH receptors. The normal testicular descent gets impeded due to close association between broad ligament and testis and vas, the mechanical effect of which prevents the testicular descent. Hence both the testes descend towards the same hemi-scrotum. Mullerian duct derivatives may also get anchored to the pelvis resulting in prevention of further testicular descent and giving rise to bilateral cryptorchidism. Infertility may result from aplasia of the epididymis or germ cell degeneration due to long standing cryptorchidism. Androgen levels, penile development and testicular histology appear normal apart from the lesions due to UDT[5-10].
AMH is member of transforming growth factor-β (glycoprotein) secreted by immature sertoli cells and lesser amount by granulosa cells of small follicles. Males lacking AMH show persistence of Mullerian derivatives with development of normal male external genitalia[1]. Expression of AMH occurs by the end of 7th embryonic week. Failure of MIF action encoded by AMH gene has been implicated in the pathogenesis of PMDS. This could be due to a defect in receptors on which MIF acts, erroneous timing in release, or complete failure of synthesis of MIF. Type-I PMDS usually occurs due to mutation in AMH gene located on chromosome 19p;13.3. So far 38 mutations have been identified. Type-II PMDS usually occurs due to mutations in AMHR2 gene, located on chromosome 12q;13. Nearly 24 types of mutations have been identified resulting in type-II PMDS. Determination of serum AMH levels with serum testosterone levels help in diagnosing different types of DSD. Mutations in AMH gene is usually suspected with presence of low or undetectable AMH levels, whereas normal serum AMH levels signifies abnormality in AMHR2 gene[5,7-10]. Gujar et al[6] reported a case of PMDS type-I, in a male of 30 years age who presented with obstructed inguinal hernia. Examination of content of the hernia sac revealed a well formed testis, uterus, cervix and upper part of vagina attached to prostate. The patient had bilateral atrophied testis, azospermia, low testosterone but a normal karyotype 46, XY.
Suresh et al[10] reported this condition in a 58 years old male with right sided indirect inguinal hernia, with no sexual dysfunction having two children. A tubular structure was identified on gross examination. CD10 and ER positivity confirmed MDS.
Early diagnosis and surgical treatment is important to prevent sexual-maturation hypertrophy of uterus leading abdominal discomfort and mass secondary to accumulation of blood. The risk of malignancy is nearly 15% in an ectopic testis in a case of PMDS, similar to that in a healthy male[11-13]. Chamarajan et al[14] reported a case of seminoma with bilateral cryptorchidic male with PMDS. Embryonal carcinoma, yolk sac tumor and teratoma can occur in patients with PMDS. As these patients usually present with a unilateral disease there is a potential chance of preserving their fertility.
PMDS is a rare entity with common presentation of hernia/cryptorchidism requiring early diagnosis and prompt treatment to prevent sterility/neoplastic transformation.
We are thankful to the Immunohistochemistry (IHC) Department of Gujarat Cancer Research Institute, Ahmedabad for providing us with their IHC facilities.
A sixteen years old boy presented with left sided undescended testis (UDT) and right sided retractile testis with urinary leak from dorsum of penis following surgery for hypospadias.
Diagnosis of UDT following ultrasonography was made and surgical exploration was performed.
Histological findings revealed presence of remnants of mullerian duct in a karyotypical male with normal testosterone levels.
Presence of mullerian structures in males is very rare and early diagnosis helps in preservation of male fertility and prevention of development of testis vanishing syndrome.
PMDS: Persistent mullerian duct syndrome; UDT: Undescended testis.
This is a well written case report of an unusual finding.
P- Reviewer: Donkov II, Ilie CP S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Josso N, Rey RA, Picard JY. Anti-müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int J Endocrinol. 2013;2013:674105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 2. | Clemente A, Macchi V, Berretta M, Morra A. Female form of persistent müllerian duct syndrome: MDCT findings. Clin Imaging. 2008;34:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Delaney DP, Kolon TF, Zderic SA. Persistent Mullerian duct syndrome associated with 47,XXY genotype. J Urol. 2004;171:852-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Renu D, Rao BG, Ranganath K. Persistent mullerian duct syndrome. Indian J Radiol Imaging. 2010;20:72-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Solanki S, Gowrishankar V, Babu MN, Ramesh S. Female form of persistent mullerian duct syndrome: Rare entity. Urol Ann. 2015;7:104-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Gujar NN, Choudhari RK, Choudhari GR, Bagali NM, Mane HS, Awati JS, Balachandran V. Male form of persistent Mullerian duct syndrome type I (hernia uteri inguinalis) presenting as an obstructed inguinal hernia: a case report. J Med Case Rep. 2011;5:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Ganguly S, Biswas RS, Saha ML, Bhattacharya S, Das S. An unusual case of inguinal hernia: Persistent mullerian duct syndrome with transverse testicular ectopia. Med Case Stud. 2011;2:54-57. [DOI] [Full Text] |
| 8. | Kumar KM, Kumar TS, Kumar MN, Pratheek KC, Krishna K. Persistent Mullerian Duct Syndrome - A Rare Anomaly. Int J Sci Stud. 2014;2:69-72. |
| 9. | Jacob M, Yusuf F, Jacob HJ. Development, Differentiation and Derivatives of the Wolffian and Müllerian Ducts. The Human Embryo. In: Dr. Shigehito Yamada, editor. InTech 2012; Available from: http://www.intechopen.com/books/the-human-embryo/development-differentiation-and-derivatives-of-the-wolffian-and-m-llerian-ducts. |
| 10. | Suresh N, Ganapathy H, Shekhar S, Prakashiny . An interesting case of Persistent Mullerian Duct Syndrome - An incidental finding. IOSR-JDMS. 2014;13:44-46. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Madhavi D. Persistent Mullerian Duct Syndrome-A Case Report. Int J Biomed Res. 2013;4:124-126. |
| 12. | Alwabari A, Parida L, Al-Salem AH. Persistent Mullerian duct syndrome: the hidden normal or abnormal anatomy and the value of laparoscopy. Ann Pediatr Surg. 2013;9:37-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Mohammadi Sichani M, Heidarpour M, Dadkhah A, Rezvani M. Persistent mullerian duct syndrome with an irreducible inguinal hernia. Urol J. 2009;6:298-300. [PubMed] |
| 14. | Chamrajan S, Vala NH, Desai JR, Bhatt NN. Persistent mullerian duct syndrome in a patient with bilateral cryptorchid testes with seminoma. J Hum Reprod Sci. 2012;5:215-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |