Published online Mar 16, 2016. doi: 10.12998/wjcc.v4.i3.76
Peer-review started: May 24, 2015
First decision: June 24, 2015
Revised: July 8, 2015
Accepted: August 20, 2015
Article in press: August 21, 2015
Published online: March 16, 2016
Processing time: 299 Days and 9.9 Hours
This case report illustrates challenging aspects of diagnosis and treatment of isolated sarcoid heart disease (SHD) and the role of cardiovascular magnetic resonance (CMR) imaging. Here, we present a previously healthy 45-year-old man, who was admitted with pericardial effusion and symptoms of acute heart failure. CMR followed by targeted left ventricular endomyocardial biopsy (EMB) revealed the diagnosis of isolated SHD. The combined use of CMR and EMB was crucial in diagnosing SHD. Furthermore, this case report demonstrates the value of CMR for monitoring response to therapy and lesion healing.
Core tip: This case report illustrates the challenging aspects of diagnosis and treatment of isolated sarcoid heart disease (SHD) and the role of cardiac magnetic resonance imaging (CMR) in diagnosis. Due to the use of CMR followed by targeted left ventricular endomyocardial biopsy the diagnosis of isolated SHD could be achieved. Most importantly, this case supports the use of CMR as an extremely useful non-invasive technique for monitoring response to therapy and lesion healing in the course of heart failure.
- Citation: Fluschnik N, Lund G, Becher PM, Blankenberg S, Muellerleile K. Fulminant isolated cardiac sarcoidosis with pericardial effusion and acute heart failure: Challenging aspects of diagnosis and treatment. World J Clin Cases 2016; 4(3): 76-80
- URL: https://www.wjgnet.com/2307-8960/full/v4/i3/76.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i3.76
Sarcoidosis is a granulomatous multisystem disorder of unknown etiology which has a wide range of manifestations affecting a variety of organs[1]. The prevalance of sarcoidosis varies with ethnicity (4.7-64/100000)[2]. Approximately 2%-7% of patients with sarcoidosis suffer from clinical cardiac manifestations[3,4]. However, several studies reveal a much higher prevalance of 20%-50% of patients with asymptomatic sarcoid heart disease (SHD) or even up to 70%-85% in autopsy studies[4,5]. Interestingly, the prevalence of isolated cardiac sarcoidosis is much higher in Japanese patients[6].
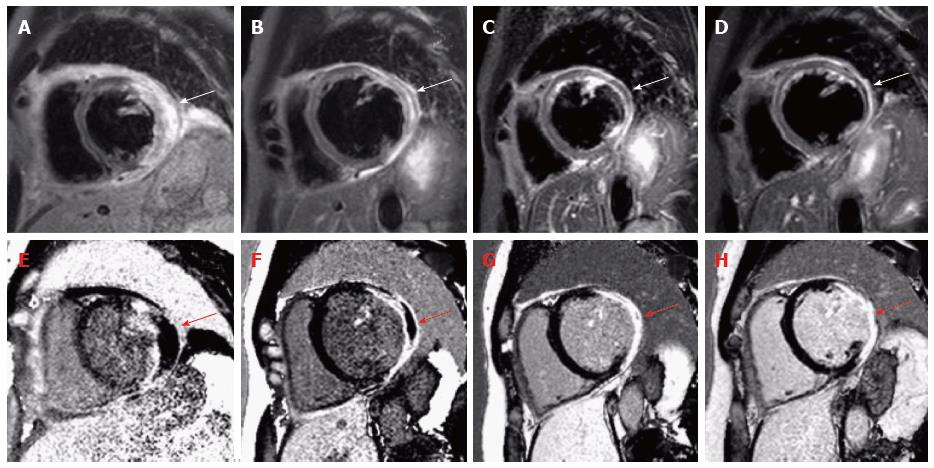
A 45-year-old man was referred to the emergency unit with syncope and temporary hemiparesis. His medical history was unremarkable besides splenectomy many years ago due to trauma. Physical examination at admission was notable for bilateral pleural effusions. Extensive neurological examinations including cranial computed tomography, brain magnetic resonance imaging (MRI), electroencephalography and lumbar puncture did not reveal any pathology. Transthoracic echocardiography revealed pericardial effusion with beginning hemodynamic relevance, possibly leading to syncope. Thus, pericardial paracentesis was performed and drained 1.8 L of hemorrhagic, sterile effusion. Subsequent laboratory findings showed increased cardiac markers (Troponin 2320 pg/mL, Creatinkinase 224 U/L, NT-proBNP 6731 ng/L) and electrocardiogram revealed abnormalities with ST-segment depression. Thus, coronary angiography was performed, which excluded coronary artery disease. Follow-up echocardiography during the next days revealed a high grade mitral regurgitation due to annular enlargement secondary to left ventricular (LV) dilatation (left ventricle end-diastolic diameter: 74 mm) and papillary muscle dysfunction, severely reduced ejection fraction (EF 30%), diastolic dysfunction and regional wall motion abnormalities of the lateral wall. Nevertheless, the etiology of the pericardial effusion and myocardial injury still remained unclear. Therefore, cardiovascular magnetic resonance (CMR) imaging was performed revealing the following findings: Severely impaired global systolic function (EF 36%), extensive edema on T2-weighted short-tau inversion recovery images as well as necrosis on late gadolinium enhancement (LGE) with a non-ischemic pattern of the lateral wall, but also the left ventricular septum (Figure 1). These findings were suspicious but not specific for sarcoidosis. Thus, we performed targeted endomyocardial biopsy (EMB) in the lateral LV wall and immunohistology revealed SHD. Interestingly, additional laboratory results were unremarkable with normal angiotensin-converting enzyme blood levels as well as normal different antibodies [anti-neutrophil cytoplasmatic antibodies (ANCAs), antinuclear antibodies, rheumatoid factors, antibodies to double-stranded DNA (anti-dsDNA), complement factors, interleukin-2 receptor]. Apart from that, computed tomography and chest X-ray excluded typically findings of pulmonary sarcoidosis such as bihiliar lymphadenopathy.
We initiated medical heart failure therapy as recommended in current guidelines[7]. After diagnosing SHD, high-dose glucocorticoid therapy with prednisone was initiated and gradually reduced over months. The follow-up-visits revealed an improved NYHA class (NYHA I-II) and clinical symptoms. Most importantly, follow-up CMRs after 1, 3 and 6 mo after initiation of the glucocorticoid therapy demonstrated resorption of edema consolidation of scar and improved systolic LV function (EF 41%) and reduced LV volumes enddiastolic volume from 290 to 276 mL, endsystolic volume from 192 to 163 mL (Figure 1). Considering the improved left ventricular function, consolidation of scar by CMR and the absence of ventricular arrhythmias on repeated Holter-ECG, we decided not to implant an internal cardiac defibrillator (ICD) for primary prevention of sudden cardiac death in this individual patient. No arrhythmic events occurred over more than one year of clinical follow-up so far.
First, this case report highlights that the diagnosis of isolated SHD is challenging. SHD has a wide range of clinical cardiac manifestations, e.g., conduction abnormalities, ventricular arrhythmias, sudden cardiac death, congestive heart failure, valve involvement, and rarely as in this patient with pericardial effusion[1]. Conventional echocardiography appears to be not sufficient to diagnose or to exclude SHD. However, speckle tracking echocardiography has been recently discussed as a novel tool to diagnose SHD. Tsuji et al[8] proposed three dimensional speckle tracking radial strain as potential method to distinguish between dilated cardiomyopathy and SHD. Others groups have reported early detection of global longitudinal strain in patients with new onset SHD[9,10]. But so far, further studies are required to evaluate strain analysis as a non-invasive method in diagnosing cardiac involvement of sarcoidosis. Furthermore, data about lesion healing and therapy monitoring with strain analysis is missing compared to CMR. Nevertheless, further studies are required to better understand the potential incremental value of these techniques.
Contrarily, CMR and targeted EMB are established tools in diagnostic evaluation of suspected SHD[2,11,12]. However, the sensitivity of EMB in SHD is only 20%-30% due to the focal appearance of non-caseating granulomas and thus false negative results, but CMR targeted EMB seems to improve the sensitivity of EMB[2,11,13]. Based on different recommendations and consensus documents, EMB should be performed in recent onset heart failure (HF) or > 3 mo duration of HF, in particular if associated with new ventricular tachyarrhythmias or second/third degree atrioventricular block or rapidly deteriorating HF[2,12].
CMR is a valuable non-invasive tool to detect SHD and to monitor therapy response as shown in this case report[2,14,15]. Conduction abnormalities and/or life threatening ventricular tachyarrhythmias are common in SHD and are related to myocardial inflammation, necrosis and/or fibrosis and scar. Consequently, implantation of ICD should be carefully evaluated in all patients suffering from SHD[16-18]. However, recent reports indicate significant rates of inappropriate shocks and device complications in patients with SHD[19,20]. As shown in this case report, CMR could be used to tailor therapy in patients with SHD. On one hand, presence and extent of scar in LGE as a measure of substrate for ventricular arrhythmia could predict risk for sudden cardiac death[21]. On the other hand, edema resorption and scar consolidation on CMR, as demonstrated in this case report, could be used to identify patients with controlled disease responding to immunosuppressive therapy. Thus, CMR seems to be helpful in risk stratification and a may be used as an adjunctive tool to guide therapy in patients with SHD. However, it is important to note that estimating risk for arrhythmia and sudden cardiac death requires careful and individual decision-making as well as informed patients.
A 45-year-old man with no significant medical history was referred to the emergency unit with syncope followed by symptoms of acute heart failure.
An unclear cardiomyopathy was found clinically accompanied by pericardial effusion with hemodynamic relevance and severe mitral regurgitation.
Myocardial infarction, dilated cardiomyopathy, giant cell myocarditis, viral myocarditis, cardiac sarcoidosis.
Laboratory findings showed increased cardiac necrosis markers (Troponin, Creatinkinase, NT-proBNP), but normal angiotensin-converting enzyme blood levels as well as normal antibodies (pANCA, cANCA, antinuclear antibodies, rheumatoid factor, anti-ds-DNA, complement factors, interleukin-2 receptor).
Cardiovascular magnetic resonance (CMR) revealed severely impaired global systolic function with extensive edema on short-tau inversion recovery images and necrosis on late gadolinium enhancement images with a non-ischemic pattern of the lateral wall, but also the left ventricular septum.
Cardiac sarcoidosis.
The authors initiated standard heart failure therapy and high-dose glucocorticoid therapy with prednisone, which was gradually reduced over months.
Only 2%-7% of patients with sarcoidosis suffer from clinical cardiac manifestations.
Sarcoidosis is a granulomatous multisystem disorder of unknown etiology which has a wide range of manifestations affecting a variety of organs.
This case highlights the challenging diagnosis and treatment of sarcoid heart disease (SHD) and the value of combining CMR with targeted endomyocardial biopsy. Moreover, this case report demonstrates the value of CMR for monitoring response to therapy in SHD.
This case report was well written and worth to be published in the journal.
P- Reviewer: Farand P, Lin GM, Pocar M S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Kim JS, Judson MA, Donnino R, Gold M, Cooper LT, Prystowsky EN, Prystowsky S. Cardiac sarcoidosis. Am Heart J. 2009;157:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in RCA: 986] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 3. | Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1469] [Cited by in RCA: 1414] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 4. | Davis RB. Hemostasis. II. The use of factor VIII concentrates in the therapy of hemophilia. Nebr State Med J. 1971;56:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204-1211. [PubMed] [Cited in This Article: ] |
| 6. | Iwai K, Sekiguti M, Hosoda Y, DeRemee RA, Tazelaar HD, Sharma OP, Maheshwari A, Noguchi TI. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11:26-31. [PubMed] [Cited in This Article: ] |
| 7. | McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803-869. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1591] [Cited by in RCA: 1835] [Article Influence: 166.8] [Reference Citation Analysis (1)] |
| 8. | Tsuji T, Tanaka H, Matsumoto K, Miyoshi T, Hiraishi M, Kaneko A, Ryo K, Fukuda Y, Tatsumi K, Onishi T. Capability of three-dimensional speckle tracking radial strain for identification of patients with cardiac sarcoidosis. Int J Cardiovasc Imaging. 2013;29:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Aggeli C, Felekos I, Tousoulis D, Gialafos E, Rapti A, Stefanadis C. Myocardial mechanics for the early detection of cardiac sarcoidosis. Int J Cardiol. 2013;168:4820-4821. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Shah BN, De Villa M, Khattar RS, Senior R. Imaging cardiac sarcoidosis: the incremental benefit of speckle tracking echocardiography. Echocardiography. 2013;30:E213-E214. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yoshida A, Ishibashi-Ueda H, Yamada N, Kanzaki H, Hasegawa T, Takahama H, Amaki M, Asakura M, Kitakaze M. Direct comparison of the diagnostic capability of cardiac magnetic resonance and endomyocardial biopsy in patients with heart failure. Eur J Heart Fail. 2013;15:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007;50:1914-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 454] [Cited by in RCA: 458] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 13. | Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, Baughman KL, Kasper EK. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Shimada T, Shimada K, Sakane T, Ochiai K, Tsukihashi H, Fukui M, Inoue S, Katoh H, Murakami Y, Ishibashi Y. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med. 2001;110:520-527. [PubMed] [Cited in This Article: ] |
| 15. | Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015;16:949-958. [PubMed] [Cited in This Article: ] |
| 16. | Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, Sweiss NJ, Nguyen DT, Aleong RG, Varosy PD. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol. 2012;23:925-929. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-62. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in RCA: 1106] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 18. | Costabel U, Skowasch D, Pabst S, Störk S, Tschöpe C, Allewelt M, Worth H, Müller-Quernheim J, Grohé C. Konsensuspapier der deutschen gesellschaft für pneumologie und beatmungsmedizin (dgp) und der deutschen gesellschaft für kardiologie – herz und kreislaufforschung (dgk) zur diagnostik und therapie der kardialen sarkoidose. Kardiologe. 2014;8:13-25. [RCA] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, Cooper JM. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012;9:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, Rosenfeld L, Mitiku TY, Cooper JM, Mehta D. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace. 2013;15:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |