Published online Aug 16, 2015. doi: 10.12998/wjcc.v3.i8.727
Peer-review started: November 26, 2014
First decision: January 20, 2015
Revised: February 6, 2015
Accepted: May 26, 2015
Article in press: May 27, 2015
Published online: August 16, 2015
Processing time: 270 Days and 8.9 Hours
To study dermatological manifestation of T-lymphoblastic lymphoma and to help clinicians in the diagnosis, we report here the case of a 75-year-old patient who presented with violaceous nodules acquired during the last 4 wk and affecting the scalp and right arm. The diagnosis of systemic lymphoma was suggested upon the appearance of cutaneous tumors, palpable lymph nodes and general symptoms including asthenia and weight-loss. The pathology features: positive immunostaining for CD3 and terminal deoxynucleotidyl transferase (TdT) and staging, led us to the final diagnosis of T-lymphoblastic lymphoma (T-LBL) with cutaneous involvement. He received a CHOP regimen as first-line treatment. Unfortunately, the patient relapsed and died 8 mo after the treatment initiation. T-LBL may be diagnosed by skin lesions. Additional immunostaining including TdT and experienced histopathologists are needed to correctly classify this aggressive disease and discuss the correct management including bone-marrow transplantation where appropriate.
Core tip: The clinical presentation of our patient with disseminated erythematic patches and infiltrated nodules suggested a diagnosis of cutaneous involvement of T-lymphoblastic lymphoma (T-LBL). Finally, histopathological examination of a skin biopsy with immunohistochemical study established the diagnosis of T-LBL. For accurate diagnosis, experienced histopathologists are needed. We wish to add this case to the current literature of T-LBL with cutaneous involvement, emphasizing the importance of a correct diagnosis and aggressive treatment.
- Citation: Ginoux E, Julia F, Balme B, Thomas L, Dalle S. T-lymphoblastic lymphoma with cutaneous involvement. World J Clin Cases 2015; 3(8): 727-731
- URL: https://www.wjgnet.com/2307-8960/full/v3/i8/727.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i8.727
T-lymphoblastic lymphoma (T-LBL) is a rare subtype of adult non-Hodgkin lymphoma (NHL) which represents almost 2% of lymphoid leukemias. This disease arises from precursor T lymphocytes and is associated with a very poor prognosis. Although skin involvement occurs in less than 20% cases it is often the first observable manifestation of the disease and leads to the correct diagnosis. By adding this case, we hope to help clinicians in this diagnosis which needs to be made fast as possible.
A 75-year-old patient was referred to our out-patient unit following the rapid onset of several reddish nodules associated with loss of weight and fatigue. His past medical history was marked by type 2 diabetes, sarcoidosis, hypertension, and prostatectomy for prostate cancer 5 years previously.
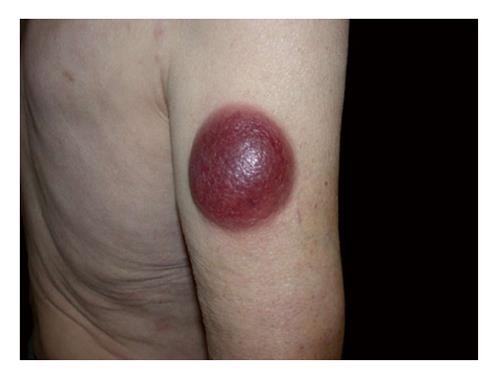
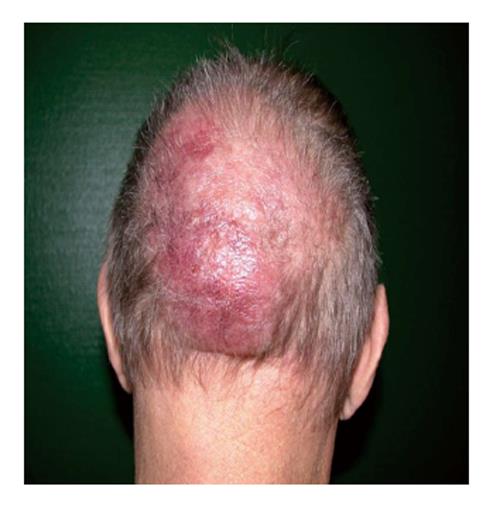
On physical examination, we observed 2 large cutaneous nodules: the first was located on his right arm (Figure 1) while the second was on the occipital area (Figure 2). The skin lesion on the arm was purplish asymptomatic and measured 10 cm on the larger diameter. The second one was a large purple painful nodule located on the occipital part of the scalp. They were both deeply infiltrated into the sub-cutaneous fat, non-pruriginous and rapidly growing. He also presented several red patches on trunk and back. On general examination we noticed bilateral palpable superficial lymph nodes on the axillary, cervical, and inguinal areas. He complained about extreme fatigue and up to 10% weight loss during the last 2 mo. Additional B-symptoms such as fever and nightly sweats were absent.
Blood samples analysis showed a lymphocytosis at 4.14 G/L, including 20% of atypical lymphocytes with irregular chromatin and a high nucleo-cytoplasmic ratio. By flux cytometry, 93% of the atypical population was CD4 positive, 50% was also CD3 positive while, of the tumoral cells, 17% aberrantly expressed CD4 and CD8.
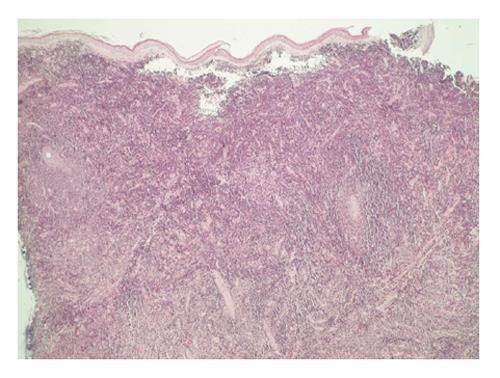
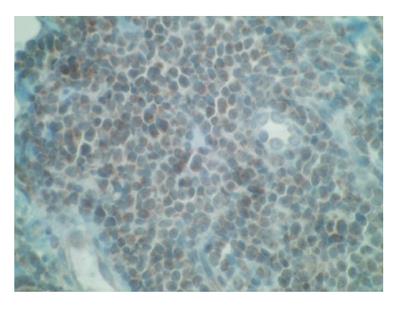
Histological examination of skin biopsy specimens from the arm lesion revealed that the dermis was infiltrated by monomorphic medium size lymphoid cells. This infiltrate was dense and diffuse infiltrating collagen bundles. The tumoral cells showed a scant cytoplasm, and an irregular prominent nucleus with dispersed chromatin and inconspicuous nucleoli. The epidermis was spared. The high number of mitotic figures rate suggested an aggressive process (Figure 3). Few mitotic figures were seen.
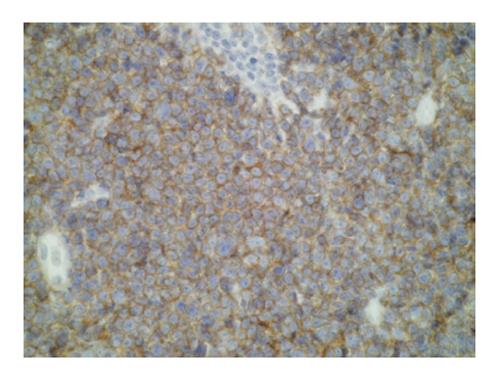
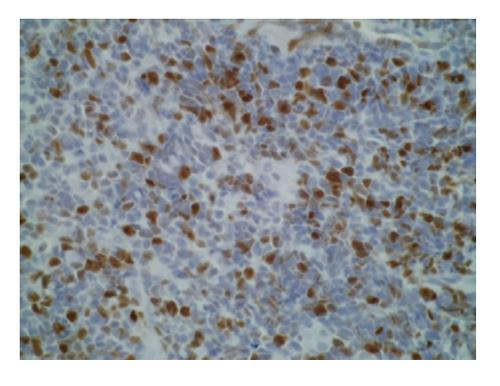
Immunostaining was performed on skin samples. The tumoral infiltrate was positive for CD3, CD5, CD4 (Figure 4), ki67 + (Figure 5) and negative for EMA (Epithelial Membrane Antigen), CD34, CD68, CD10, CD30, CD20, CD79, CD23, CD56, CD8, and myeloperoxidase. Terminal deoxynucleotidyl transferase (TdT) marker, a specialized DNA polymerase expressed in immature, pre-B, pre-T lymphoid cells, and acute lymphoblastic leukemia/lymphoma cells, was found positive on paraffin embedded biopsy (Figure 6). Bone marrow aspiration showed an identical intramedullar lymphocytic T-cell proliferation, thus resulting in a diagnosis of lymphoblastic lymphoma. A total body computed tomography-scan showed multiple tumoral lymph nodes on cervical, axillar, inguinal and deeper areas.
The diagnosis of T-LBL CD4+, CD56- with cutaneous involvement and leukemic involvement was made. The Ann Arbor stage was IV B and E.
The patient was then referred to the haematology department. This patient was not eligible for bone marrow transplantation. Because of the aggressiveness of the disease, a CHOP regimen (cyclophosphamide, doxorubicin, vincristin, prednisone) was immediately started as first-line treatment. Despite a short-term partial response, the patient relapsed after the fourth cycle of chemotherapy and was subsequently included in a Holoxan (ifosfamide) Vepeside (etoposide) therapeutic protocol. After 3 cycles of this chemotherapy a clinical remission occurred and because of poor hematologic tolerance (grade 4 leucopenia and thrombopenia) the treatment was discontinued. The patient relapsed and was admitted into a palliative care strategy. He eventually died 8 mo after the initial diagnosis.
T-LBL is a rare subtype of adult non-Hodgkin lymphoma with an incidence representing approximately 2% of NHL cases[1]. Lymphoblastic lymphoma predominates in young adults and teenagers with a median age at diagnosis of 20 years. It has a poorer prognosis compared to B-LBL. In adults the overall survival rate at 5 years is 26%[2-6]. Prognostic factors for adult T–LBL have not so far been determined.
This disease is the malignant counterpart of the precursor T-cell lymphocytes. The lymphoblasts infiltrate lymph nodes or extranodal structures, especially the bone marrow, spleen, and central nervous system[3]. It is characterized by male predominance, high rate of lactate dehydrogenase, III or IV Ann Arbor staging and mediastinal involvement[2] (Table 1). Typically, patients with lymphoblastic lymphoma are diagnosed with painless lymphadenopathy, splenomegaly, neurologic deficits or testicular masses[2].
| Patient | Sex/age | Staging | Cutaneous involvement | Extracutaneous lesions |
| 1[2] | M/16 | Stage 4 | Subcutaneous nodule on the left side of neck | BM involvement, lymph nodes |
| 2[2] | M/20 | Stage 4 | Multiple scalps nodules | Prostate involvement |
| 3[2] | M/25 | Stage 4 | Multiple subcutaneous nodules on left side of face, scalp, and chest wall | BM involvement, lymph nodes, scrotal involvement |
| 4[2] | M/17 | Stage 4 | Subcutaneous nodules on left side of neck and abdomen | Mediastinal mass, BM involvement, lymph nodes |
| 5[2] | F/25 | Stage 4 | Multiple subcutaneous nodules on right breast, right side of neck, chest wall, and both legs | BM involvement, lymph nodes |
| 6[2] | M/39 | Stage 4 | Multiple nodule on scalp | Mediastinal mass, lymph nodes, BM, bone, left kidney, left pleura involvement |
| 7[2] | M/22 | Stage 4 | Multiple subcutaneous nodules on back | Mediastinal mass, lymph nodes, tonsil, nasopharyngeal involvement |
| 8[7] | F/24 | Stage 4 | Multiples nodules on malar regions of face, breast, trunk | Lymph nodes, conjunctiva |
| 9[7] | M/25 | Stage 4 | Nodule on scalp | Mediastinal mass |
| 10[8] | M/8 | Stage 1 | Nodule on abdominal wall | None |
| 11[4] | M/65 | Stage 4 | Multiples nodules on lower extremities and abdomen | Mediastinal mass, lymph nodes and BM |
| 12[9] | F/29 | Stage 4 | Multiples nodule face, breast, thoraco - mammary region, abdomen, tights | Lymph nodes, BM |
| 13 case reported | M/75 | Stage 4 | Nodules on scalp and right arm and multiples patches on trunk | Lymph nodes, BM |
Involvement of the skin in T-LBL is rare, although the exact frequency at which this occurs has not been calculated[4]. In the majority of reported cases, the cutaneous involvement is secondary or concomitant to bone marrow or lymph node spreading[6]. Skin involvement is more common in association with B-LBL.
Diagnosis is commonly made thanks to an excisional node biopsy or exploration of extranodal sites. Typically, histology with hematoxylin-eosin coloration shows a monomorphous dermis-infiltrate of small to medium-sized lymphoid cells with scant cytoplasm, moderately condensed to dispersed chromatin and prominent nucleus with inconspicuous nucleoli[5]. Epidermotropism is not present and the grenz zone is located beneath the epidermis. In some areas, the tumor cells can be arranged in a mosaic-like pattern[4]. However, diagnosis is not made by Hematoxylin-Eosin sections alone; immunohistochemical studies are also needed to diagnose T-LBL. T-LBL is confirmed when lineage markers for B-cells (CD20) and NK cells (CD56) are negative, and those for precursor T cells (CD3 and CD5) are positive, whilst expression of ki67 proves malignancy. TdT expression is seen only in lymphoblastic lymphoma (B-LBL and T-LBL) contrary to the mature lymphoma[5]. A recent study based on cytogenetic analysis and gene expression profiling proved that primary cutaneous T-LBL is characterized by a gain of 1p36.33-p22.1 in the early stages and a large number of chromosome losses/gain in the later stage. The gain of 1p36.33-p22.1 could be an interesting marker to perform an earlier diagnosis of primary cutaneous T-LBL[9].
Diagnosis is also made on bone marrow biopsy, with lymphoblasts which may comprise up to 25% of marrow elements. If lymphoblasts involve more than 25% of the marrow, patients are arbitrarily diagnosed with acute lymphoblastic leukemia (ALL).
Indeed, conversely to cutaneous B-LBL which typically presents as a unique lesion located in neck or head area, T-LBL develop multiple skin lesions on back, chest, legs and breasts to the same extent as the head and neck areas[2]. This clinical presentation must be differentiated from the blastic plasmacytoid dendritic cell neoplasm presentation which can be quite similar. It is a disease with frequent skin involvement appearing as widespread, purplish, dermal nodules localized on the trunk, limbs or head. Usually, as in T-LBL, patients progress rapidly with involvement of bone marrow, peripheral blood, lymph nodes and extranodal sites. Diagnosis can be made from the immunohistochemical analysis of tumor cells when TdT and T-cell markers (CD3) are negative and CD4 and CD56 positive[10].
Various treatments have been applied to LBL in adult: protocols for high-grade NHL such as CHOP, CHOEP, or treatments according to a regimen for ALL, and autologous stem cell transplantation (SCT)[1]. The major challenge of local disease control is the effective treatment of mediastinal tumors. It is now believed that ALL chemotherapy regimens are more appropriate for LBL[11]. It has been shown that a hybrid NHL/ALL chemotherapy protocol followed by consolidation with SCT appears superior to the use of chemotherapy alone[11].
The clinical presentation of our patient with disseminated erythematic patches and infiltrated nodules suggested a diagnosis of cutaneous involvement of T-LBL. Finally, histopathological examination of a skin biopsy with immunohistochemical study established the diagnosis of T-LBL. For accurate diagnosis, experienced histopathologists are needed.
We wish to add this case to the current literature of T-LBL with cutaneous involvement, emphasizing the importance of a correct diagnosis and aggressive treatment.
We thank Sarah Kabani for editorial assistance.
The disease arises from precursor T lymphocytes and has a poor prognosis.
The clinical presentation of the skin involvement is large purplish skin nodules.
The blastic plasmacytoid dendritic cell neoplasm but the Cd4 and CD56 are positive.
Positive terminal deoxynucleotidyl transferase (TdT) marker, positive precursors T cell markers (CD3 and CD5) and histology make the diagnosis.
The pathological diagnosis of skin involvment of T-lymphoblastic lymphoma (T-LBL) is made by histology with hematoxylin-eosin coloration which commonly shows a monomorphous dermis-infiltrate of small to medium-sized lymphoid cells with scant cytoplasm, moderately condensed to dispersed chromatin and prominent nucleus with inconspicuous nucleoli.
Protocols for high-grade non-Hodgkin lymphoma (NHL) like CHOP CHOEP or stem cells transplantation.
Some related case has been reported by Lee et al[2] (Precursor B- or T-LBL presenting with cutaneous involvement: a series of 13 cases including 7 cases of cutaneous T-LBL).
Tdt marker is a specialized DNA polymerase expressed in immature pre-B, pre-T lymphoid cells and acute lymphoblastic leukemia.
Importance of a correct diagnosis for a fast treatment.
The authors have performed a good study, the manuscript is interesting.
P- Reviewer: Akbulut S S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Hoelzer D, Gökbuget N, Digel W, Faak T, Kneba M, Reutzel R, Romejko-Jarosinska J, Zwolinski J, Walewski J. Outcome of adult patients with T-lymphoblastic lymphoma treated according to protocols for acute lymphoblastic leukemia. Blood. 2002;99:4379-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 167] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Lee WJ, Moon HR, Won CH, Chang SE, Choi JH, Moon KC, Lee MW. Precursor B- or T-lymphoblastic lymphoma presenting with cutaneous involvement: a series of 13 cases including 7 cases of cutaneous T-lymphoblastic lymphoma. J Am Acad Dermatol. 2014;70:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Soslow RA, Baergen RN, Warnke RA. B-lineage lymphoblastic lymphoma is a clinicopathologic entity distinct from other histologically similar aggressive lymphomas with blastic morphology. Cancer. 1999;85:2648-2654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Chimenti S, Fink-Puches R, Peris K, Pescarmona E, Pütz B, Kerl H, Cerroni L. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26:379-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Terada T. TDT (-), KIT (+), CD34 (+), CD99 (+) precursor T lymphoblastic leukemia/lymphoma. Int J Clin Exp Pathol. 2012;5:167-170. [PubMed] |
| 6. | Schmitt IM, Manente L, Di Matteo A, Felici F, Giangiacomi M, Chimenti S. Lymphoblastic lymphoma of the pre-B phenotype with cutaneous presentation. Dermatology. 1997;195:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Sander CA, Medeiros LJ, Abruzzo LV, Horak ID, Jaffe ES. Lymphoblastic lymphoma presenting in cutaneous sites. A clinicopathologic analysis of six cases. J Am Acad Dermatol. 1991;25:1023-1031. [PubMed] |
| 8. | Prasad PG, Cyriac S, Sagar TG, Rathnam K. T lymphoblastic lymphoma relapsing in skin - a rare clinical scenario. Indian J Hematol Blood Transfus. 2010;26:19-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Vezzoli P, Novara F, Fanoni D, Gambini D, Balice Y, Venegoni L, Paulli M, Crosti C, Berti E. Three cases of primary cutaneous lymphoblastic lymphoma: microarray-based comparative genomic hybridization and gene expression profiling studies with review of literature. Leuk Lymphoma. 2012;53:1978-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Julia F, Petrella T, Beylot-Barry M, Bagot M, Lipsker D, Machet L, Joly P, Dereure O, Wetterwald M, d’Incan M. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, Lavoie JC, Nantel SH, Nevill TJ, Shepherd JD. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol. 2007;18:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |