Published online Aug 16, 2015. doi: 10.12998/wjcc.v3.i8.675
Peer-review started: March 3, 2015
First decision: April 27, 2015
Revised: May 15, 2015
Accepted: June 1, 2015
Article in press: June 2, 2015
Published online: August 16, 2015
Processing time: 171 Days and 21.3 Hours
The manuscript describes the efficacy of a new skin closure system (ZipLine™) for wound closure after pacemaker/implantable cardioverter defibrillator surgery. The system is particularly useful when wound healing is difficult with traditional methods and in patients at high risk for surgical site infections (SSIs). This skin closure option is easy and quick to apply and remove, and produces excellent cosmetic results. Although it is associated with a minimal expense upcharge, the benefits, including the potential for decrease in SSI, make it attractive and worth considering for skin closure in device patients, particularly those at increased risk of complications.
Core tip: A new skin closure system (ZipLine™) is available for wound closure after pacemaker/implantable cardioverter defibrillator surgery. The system is particularly useful when wound healing is difficult with traditional methods and in patients at high risk for surgical site infections (SSIs). This skin closure option is easy and quick to apply and remove. The benefits, including the potential for decrease in SSI, make it attractive and worth considering for skin closure in device patients, particularly those at increased risk of complications.
- Citation: De Maria E. New skin closure system facilitates wound healing after cardiovascular implantable electronic device surgery. World J Clin Cases 2015; 3(8): 675-677
- URL: https://www.wjgnet.com/2307-8960/full/v3/i8/675.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i8.675
Cardiovascular implantable electronic device (CIED) infections are dramatically growing; estimated incidence is between 1% and 2% of all procedures, with a higher risk for implantable defibrillators and biventricular systems. Prevention is of the utmost importance and should be approached with the same strategy for surgical site infections (SSIs)[1]. The method of skin closure may be a contributing factor toward avoiding such complications; insufficient and/or delayed closure of the wound is a well recognized risk factor for subsequent infections[1]. Options for skin closure include subcuticular reabsorbable suture (braided versus monofilament), surgical “glue”, and staples[2]. Closing surgical incisions with traditional sutures and staples increases the risk of SSIs compared to tape-based closure[3,4]. Sutures and staples hold the skin where they pass through it, creating high stress points that can cause ischemia, pain, irritation, edema, slow wound healing and promote scar formation.
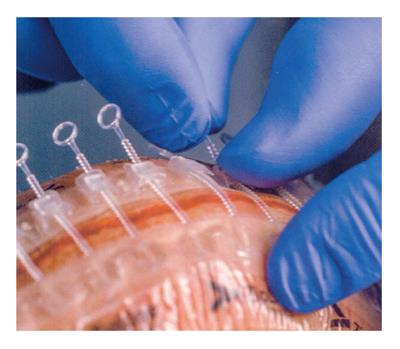
Recently a new “non invasive” device for skin closure (Zip™ Surgical Skin Closure, ZipLine Medical, Inc., Campbell, CA, United States) was approved for low tension, non-infected, surgical wounds. It is a sterile single-use system with two self-adhesive hydrocolloid pressure-sensitive strips linked with individually adjustable self-locking fasteners (Figure 1). This device increases tensile strength of a wound faster than sutures; the non-uniform distribution of closure forces associated with sutures allows immature collagen fibers to remain in a disorganized arrangement for a longer time, while in a wound closed with an adhesive tape-based closure, stress is uniformly applied to the collagen fibers which cross the wound causing rapid fiber orientation and increased tensile strength[2,3]. In addition, necrosis of tissue, needle puncture marks and suture scarring are eliminated, leading to better cosmetic results and reduced closure time, potentially reducing SSI risk[5].
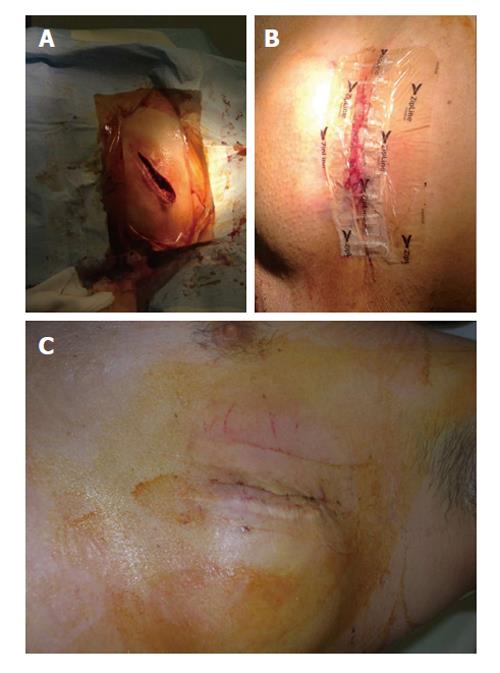
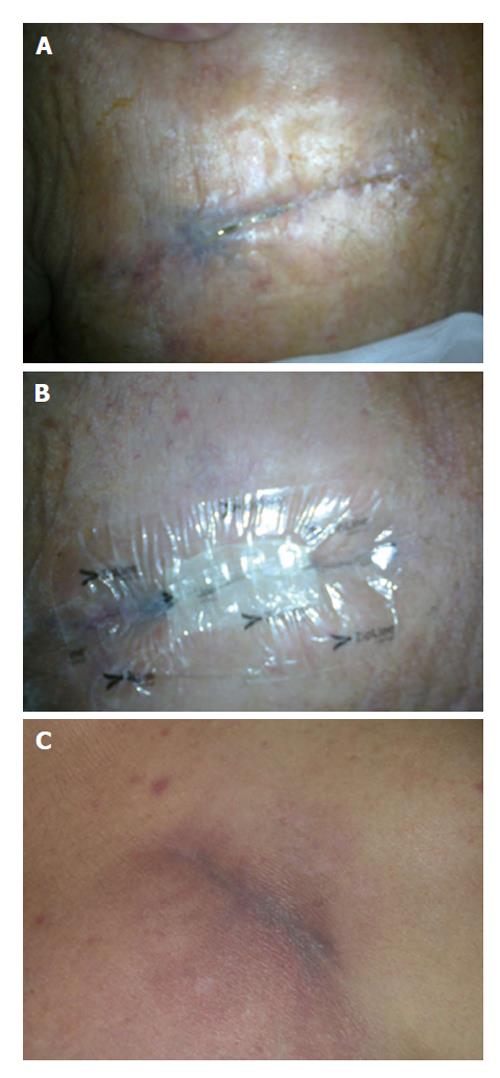
Here we present two cases of CIED surgery wounds treated with Zip™ system. In the first case the system was placed as first-line option after the replacement of a subcutaneous defibrillator [S-implantable cardioverter defibrillator (ICD)] due to battery depletion; the pocket was localized near the left anterior axillary line, a zone at risk for suboptimal wound closure (Figure 2). The second patient was an obese diabetic on hemodialysis who had an incomplete wound healing three weeks after a transvenous ICD placement (pocket in the right subclavicular area): the skin remained partially “opened” after having used all “traditional” methods for closure, so a Zip™ device was placed as a “rescue” (Figure 3). In both cases subcutaneous tissues had already been closed with monofilament suture for pocket and subcuticular layer, leaving a < 5 mm incision gap (importantly the device does not replace subcutaneous or other deep, tension-reducing sutures). After 14 d ZipLine was removed obtaining a complete wound healing without further problems in both patients, even at 6 mo follow up.
Although this system is associated with a minimal expense upcharge, the benefits (including the potential for decrease in SSI) make it attractive and worth considering for skin closure in device patients, particularly those at increased risk of complications and delayed healing of the wound: patients with heart failure, diabetes, renal insufficiency, cancer, immunodeficiencies, long-term corticosteroid use, high bleeding risk, longer and more complex procedures. Future studies will be needed to determine if and to what percentage this new device will reduce the incidence of SSI.
P- Reviewer: Amiya E, Cebi N, Celikyurt YU, Kato M, Rauch B, Sakabe K S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | De Maria E, Diemberger I, Vassallo PL, Pastore M, Giannotti F, Ronconi C, Romandini A, Biffi M, Martignani C, Ziacchi M. Prevention of infections in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med (Hagerstown). 2014;15:554-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Moy RL, Lee A, Zalka A. Commonly used suturing techniques in skin surgery. Am Fam Physician. 1991;44:1625-1634. [PubMed] |
| 3. | Iavazzo C, Gkegkes ID, Vouloumanou EK, Mamais I, Peppas G, Falagas ME. Sutures versus staples for the management of surgical wounds: a meta-analysis of randomized controlled trials. Am Surg. 2011;77:1206-1221. [PubMed] |
| 4. | Spencker S, Coban N, Koch L, Schirdewan A, Mueller D. Comparison of skin adhesive and absorbable intracutaneous suture for the implantation of cardiac rhythm devices. Europace. 2011;13:416-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Henretig FM, King C. Textbook of Pediatric Emergency Procedures, Chapter 111 Laceration Repair. Baltimore. 1997;1159. |