CASE REPORT
This 70-year-old right-handed woman with an unremarkable past medical history was incidentally diagnosed 4 years prior with two small tandem ANs on the right anterior inferior cerebral artery (AICA) feeding a small right cerebellopontine angle AVM. The patient was referred to our hospital for further evaluation and treatment and despite informed consent regarding the potential risks of subsequent hemorrhage and recommendations for microsurgery, Gamma Knife stereotactic radiosurgery, or endovascular embolization, or other interventions, she and her family chose conservative outpatient observation.
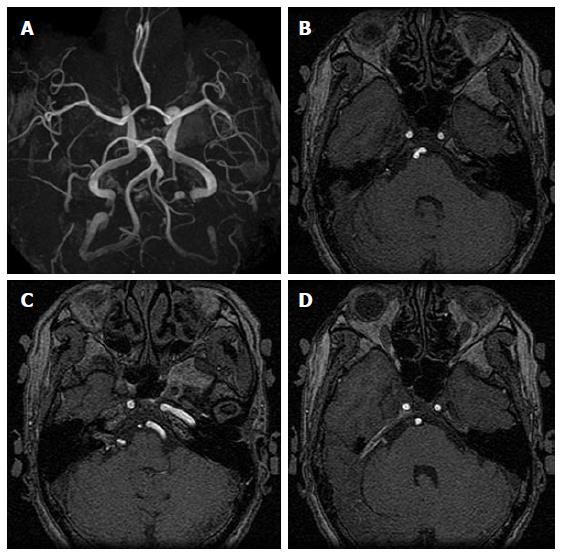
Digital subtraction angiography (DSA) was recommended for primary evaluation, however based on the invasiveness, potential complications, and inconvenience, she declined this modality in favor of three-dimensional computed tomography angiography (3D-CTA) and magnetic resonance angiography (MRA) for initial diagnostic evaluation followed by biannual and thereafter annual serial magnetic resonance imaging (MRI) (Figure 1). Dedicated informed consent was routinely offered to the patient and family including an explanation of the cumulative bleeding risks and recommendations for aggressive surgical or alternative treatment options. However the patient declined treatment and was followed on an outpatient basis until she presented to our outpatient clinic with a complaint of severe headache, nausea and vomiting of several days duration. The neurological exam was unremarkable with no apparent cranial nerve deficits. Head computed tomography (CT) did not demonstrate any evidence of bleeding, however, high intensity signals only in the quadrigeminal cistern on T1-weighted MRI were observed, suggesting early subacute SAH (Figure 2). Fluid attenuated inversion recovery imaging was not performed. MRA source imaging and magnetic resonance cisternography demonstrated a small (11.4 mm × 4.2 mm) relatively compact nidus located in the cerebellopontine angle around the cisternal portion of the facial and vestibulocochlear nerves (cranial nerves VII and VIII, respectively) with extension into the internal auditory canal (Figure 3). On the arterial phase of DSA the right AICA was identified as the predominant feeder with drainage into the right petrosal vein, superior petrosal sinus, and cavernous sinus (Figure 4). Based on the lack of any significant cranial nerve deficits, the occurrence of either minor AVM leakage, hypertensive venous bleeding, or subarachnoid hemorrhage from AN rupture was postulated. Because of the symptomatic nature of this Spetzler-Martin grade III AVM (S1V1E1) with two feeding artery aneurysms, and after a comprehensive explanation of the risks and benefits of the various treatment options, including the risks of microsurgical neurological sequelae, the persistent delayed risk of bleeding following radiation therapy, and the possible risk of recurrence or other complications following endovascular embolization, the patient and family agreed to a recommendation of microsurgical treatment.
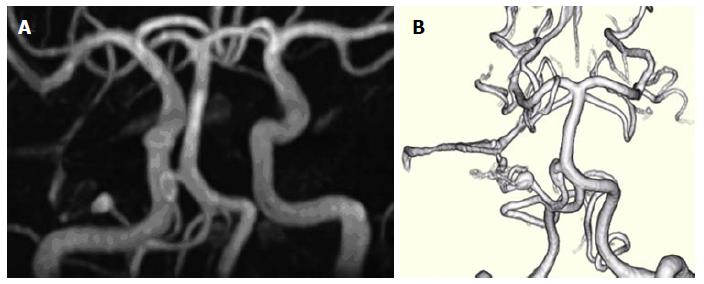
Figure 1 Three-dimensional time-of-flight magnetic resonance angiography.
A: Three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) four years prior to admission; B: Volume rendering 3D-TOF-MRA study 1 year prior to admission, demonstrating two unruptured prenidal feeder artery aneurysms, one 8 mm × 3 mm fusiform proximal anterior inferior cerebral artery (AICA) aneurysm near the takeoff of the basilar artery and one 4.5 mm × 3 mm elliptical distal pedicle AICA aneurysm located at the entrance to the main inflow area of a small inconspicuous vascular nidus.
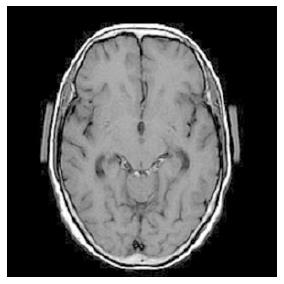
Figure 2 On admission, this 70-year-old woman presented with a severe headache, nausea and vomiting of several days duration.
T1-weighted magnetic resonance imaging demonstrated high intensity signals only in the quadrigeminal cistern, suggestive of early subacute subarachnoid hemorrhage.
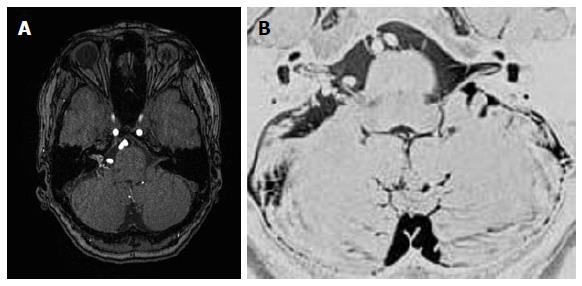
Figure 3 Magnetic resonance angiographic source imaging (A) and magnetic resonance cisternography (B) reveal a small (11.
4 mm × 4.2 mm) relatively compact nidus located in the cerebellopontine angle at the cisternal portion of the cranial nerves VII and VIII with extension into the internal auditory canal.
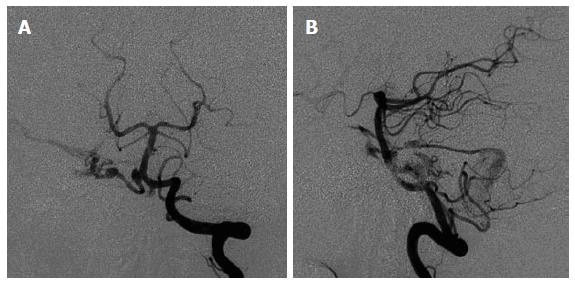
Figure 4 Anterioposterior (A) and lateral (B) views of cerebral angiogram for this patient showing the right anterior inferior cerebral artery as the predominant feeder supplying a small nidus with drainage into the right petrosal vein, superior petrosal sinus, and cavernous sinus [Spetzler-Martin grade III (S1V1E1)].
Surgical procedure
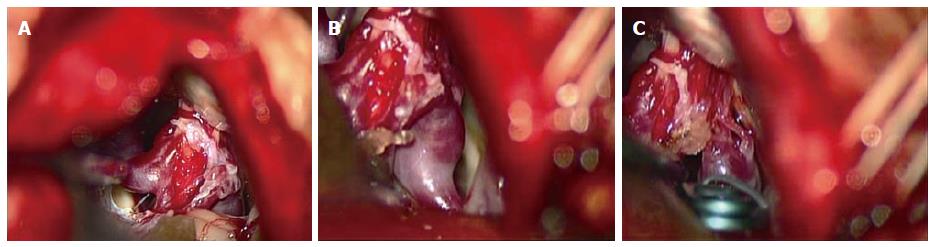
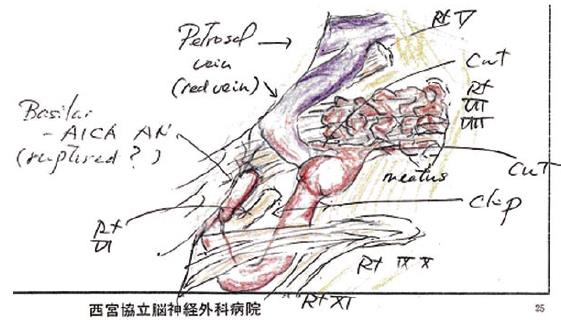
The patient was placed in the right lateral oblique position and a right retrosigmoid craniotomy was performed. After exposing the right lateral cistern and cerebellopontine angle, the right facial and vestibulocochlear nerves (cranial nerves VII and VIII, respectively) were identified followed by confirmation of the right lower cranial nerves (cranial nerves IX, X, and XI), the right AICA, proximal basilar-AICA bifurcation, and surrounding trigeminal and abducens nerves (cranial nerves V and VI, respectively). The right AICA was determined to be the predominant feeder supplying a small peripheral nidus buried within the right cranial nerve VIII at the meatus of the internal auditory canal with venous drainage into an engorged red petrosal vein (Figure 5A and B). Close inspection revealed a mid-sized fusiform AN at the proximal AICA near the takeoff from the basilar artery and a small distal pedicle feeding artery AN at the inflow area of the nidus. Xanthochromic cerebrospinal fluid was observed with a slight predominance near the proximal AICA AN, however there was no clear evidence of a rupture point from either of the ANs, nidus, or venous drainers. Based on the preoperative imaging studies, the nidus was suspected to extend into the auditory canal, and complete resection was not attempted because of the anticipated high risk of auditory nerve injury. Instead a feeder clip was applied to the AICA which demonstrated normalization of color from red to blue in the right petrosal vein. However, intraoperative microvascular doppler ultrasonography and indocyanine green video angiography confirmed the presence of persistent flow within the AVM despite temporary clipping, suggesting the existence of collateral supply from the external carotid artery system or other sources. Definitive clipping between the proximal and distal AICA ANs followed by partial electrocoagulation of both the nidus and the predominant entry area to the nidus were performed (Figure 5C) with the intention of alleviating hemodynamic stress on the remaining nidus and proximal AN. Figure 6 shows an overall intraoperative schematic illustration. Although auditory brainstem response monitoring remained normal throughout all procedures, preventative intravenous corticosteroids were administered postoperatively.
Figure 5 Intraoperative microscopic view.
A: Intraoperative microscopic view showing a right retrosigmoid craniotomy in the a right lateral oblique position which exposed a small nidus buried within the right cranial nerve VIII at the meatus of the internal auditory canal; B: View showing distal right anterior inferior cerebral artery feeding aneurysm, nidus-VIII cranial nerve complex, and draining petrous vein; C: Definitive clip application proximal to the aneurysm and partial electrocoagulation of the nidus and the inflow area just proximal to the nidus was performed.
Figure 6 Intraoperative schematic illustration depicting anatomical structures and definitive clipping between the proximal and distal feeding anterior inferior cerebral artery aneurysms and partial electrocoagulation of both the nidus and the main inflow area to the nidus.
Postoperative course
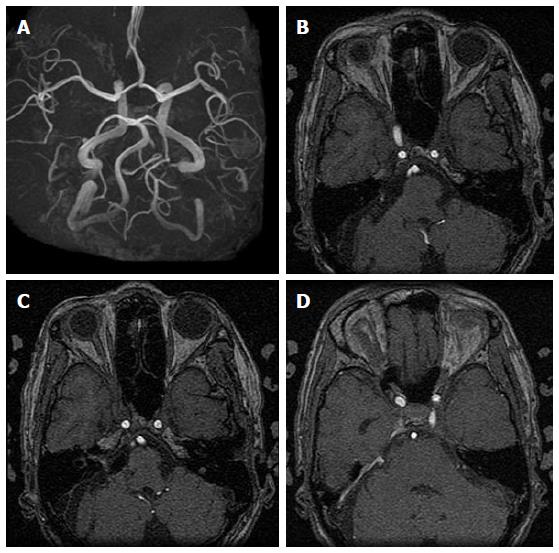
The patient had an uneventful postoperative course with follow-up clinical and head CT examinations every 2 to 3 mo for a period of 2.5 years. Biannual follow-up outpatient MRI and MRA source imaging during that period showed clip artifact at the entrance to the internal auditory canal with apparent disappearance of proximal and distal right AICA aneurysms, and a prominent right superior petrosal sinus, indicative of residual shunt flow (Figures 7 and 8 compare preoperative and postoperative changes). The patient experienced a transient right hearing deficit, diplopia, and vertigo which almost completely resolved on discharge. More extensive diagnostic imaging and treatment options including Gamma Knife radiosurgery were under consideration pending the onset of radiological regrowth or symptomatic recurrence.
Figure 7 Preoperative three-dimensional time-of-flight magnetic resonance angiography showing proximal and distal right anterior inferior cerebral artery aneurysms (A), and magnetic resonance angiographic source imaging showing proximal right anterior inferior cerebral artery aneurysm (B), distal right anterior inferior cerebral artery aneurysm with relatively strong internal auditory canal nidus signal intensity (C), and right superior petrosal sinus venous shunt (D).
Figure 8 Postoperative three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) and magnetic resonance angiographic source imaging showing anterior inferior cerebral artery (AICA) stump with disappearance of proximal and distal right AICA aneurysms (A, B), decreased intensity of right AICA and seemingly disappearance of proximal aneurysm, clip artifact at the entrance to the internal auditory canal and decreased intensity of intracanicular nidus (C), and persistence of prominent right superior petrosal sinus venous shunt (D).
DISCUSSION
Intrinsic AVMs limited exclusively to the cranial nerves are extremely uncommon although there have been many reports of AVM rupture resulting in damage to nearby cranial nerves or indirect feeder compression of cranial nerves with associated symptoms manifesting in various conditions such as optic nerve apoplexy[1-6], oculomotor nerve ophthalmoplegia[28], trigeminal neuralgia[7,8,10-18,20,21,23,24], hemifacial spasm[29,30], glossopharyngeal neuralgia[31], and hypoglossal nerve paresis[32]. Several rare reports of AVMs originating from within the trochlear nerve[33] and trigeminal nerve[9,19,22] have also been documented. Maher et al[19] proposed an embryological basis for explaining the occurrence of AVMs arising in cranial nerves such as the trigeminal nerve, whereby normally migrating fetal axons carry precursor AVM cells toward an ectopic cranial nuclei location during the 5th to 6th weeks of embryogenesis.
To date, there has been only one case report of an AVM located within the auditory nerve bundle which presented with SAH yet this case involved complete sensorineuronal hearing loss[27]. Because of the severe functional deficit from onset in that patient, total removal of both the nidus and nerve remnant were performed. Interestingly, in many of the cases of intrinsic trigeminal or trochlear AVMs as well as in our case, the patients presented with symptoms unrelated to cranial nerve dysfunction from direct AVM rupture[19,33], or symptoms were not caused by the nidus itself but by neural compression from surrounding vessels such as the feeding superior cerebellar artery[17,18,20,22] or impingement from other vessels at the trigeminal nerve root entry zone[15,16]. In a study by Sumioka et al[22] a review of over 600 cases of trigeminal neuralgia were identified to be caused by vascular compression while only a small minority showed no evidence of vascular involvement. In these non-vascular cases, symptoms of trigeminal neuralgia were postulated to be derived from either the twisting or “tilting” by mechanical compressive forces, thickened arachnoid, or the direct effects of embedded AVMs.
In addition, many of the cases of chiasmal or optic nerve apoplexy due to intrinsic AVMs (including cavernous malformations) were typically diagnostically labeled as angiographically occult and only after performing high-resolution MRI were these cryptic micro-AVMs identified[3]. MRI has also been considered to offer a considerable advantage over standard angiography both for reducing complications and procedural related morbidity as well as providing detailed delineation of neurovascular structural relationships crucial for making treatment decisions during the preoperative and postoperative periods[34]. Moreover, in a recent report of the largest surgical series of radiological and clinical follow-up of AVM recurrence after surgery, MRI with and without contrast administration was recommended as the initial method for monitoring recurrence, with subsequent performance of digital subtraction angiography only in the event of suspected recurrence[35].
Likewise, a review of the recent literature seems to indicate a more palliative, minimally invasive trend for the treatment of cranial nerve AVMs. Sumioka et al[22] have suggested that the goal of treating intrinsic AVMs of the trigeminal nerve is generally symptomatic treatment, with prevention of rebleeding and preservation of cranial nerve function. Although treatment of cranial nerve cavernous malformations has been shown to be relatively successful with a lack of major associated complications to the surrounding neurovascular structures, direct microsurgical AVM resection in and around the optic nerve as well as other cranial nerves has typically been associated with significant damage to the nerve itself as well as surrounding microvasculature structures[9,18,22,23]. Indeed Sasagawa et al[5] have suggested that curative surgical treatment of optic nerve AVMs is considered nearly impossible, while other reports have shown that microsurgery can be used diagnostically as well as theraputically via limited surgical treatment such as optic nerve decompression or by subtotal operation for prevention of AVM growth or rebleeding[1]. As an example, an 8-year-old boy who presented with optic nerve apoplexy was found to have an AVM of the optic nerve and chiasm, but was treated by a diagnostic craniotomy alone without resection[5]. In another case of trigeminal neuralgia caused by an intrinsic trigeminal nerve AVM, complete resection of the AVM was judged to be too risky and only partial microsurgical nidus coagulation, transposition of the offending superior cerebellar artery, and subsequent Gamma Knife radiosurgery was performed for eventual complete radiological obliteration[22].
In our case the choice of treatment took into consideration both the angioarchitectural and clinical features as well as patient preference for a less aggressive option involving minimal risk. Due to the presence of symptomatic SAH presumed to be related to AN rupture and based on the high risk of nerve injury from complete AVM extirpation, a more palliative flow reduction strategy of feeder artery electrocoagulation at the inflow area of the nidus, effective feeder AN trapping, and partial electrocoagulation of the nidus was performed. Although intraoperative findings showed a slight predominance of xanthochromic cerebrospinal fluid near the proximal AICA AN, which was suggestive of having a higher likelihood of being the causative source of bleeding, because of the risk of damaging the abundant small perforators in the basilar-AICA takeoff area, a more distal obliteration point closer to the main inflow region of the AVM was chosen. Despite the seemingly unconventional nature of this method, in principle, we preliminarily followed standard microsurgical AVM treatment protocol including coagulation of the major superficial feeding artery at a location as close as possible to the point of feeder entry into the nidus[34]. And according to current recommendations for treatment of coexisting intracranial ANs, we attempted to diminish the hemodynamic stress by obliteration of high flow shunting, a variation on a technique which has been shown to result in either regression or disappearance of the prenidal AN[34,36]. However, by placing a clip distal to the proximal AN, there could be a risk of subsequent increased hemodynamic stress and hemorrhage for this aneurysm, especially due to limited distal outflow. Careful follow-up diagnostic studies and consideration of endovascular or other treatment modalities would be recommended. Finally, the observation of a temporary postoperative hearing disturbance may possibly be explained by intraoperative manipulation of the cochlear nerve. However, the possibility of vascular compromise with compensatory extracarotid flow could explain the recovery in hearing, yet this mechanism would be expected to occur over a longer period of time.
Classically there has been caution regarding the potential increased risks of bleeding from incomplete, partial, or subtotal AVM resection[8], as well as recent concerns of bleeding after palliative embolization[34]. In contrast, embolization designed to reduce flow and decrease risk of rupture has been indicated in various conditions, including treatment of associated ANs and large inoperable AVMs, or “inoperable” vascular lesions with recurrent hemorrhage, or locations in eloquent regions, where multimodality approaches of preoperative embolization combined with microsurgical extirpation have been considered. However, there is no evidence in support of such empirical methods and ultimately treatment must be individualized[34,37]. Recently, the Barrow vascular group has found moderate success for treatment of vertebrobasilar aneurysms via low-flow bypass and flow reduction via complete or partial basilar artery vessel occlusion or distal vertebral artery occlusion[38]. Although our procedure as well as the angioarchitecture of the lesions differed in many ways from the technique used in that study, both lesions resided near critical neurovascular structures and in principle, both involved application to some degree of a procedure designed to reduce flow to the inflow vessel of the aneurysm. Another study from the same institution achieved satisfactory long-term outcome using a technique of subtotal nidus resection for effective devascularization of glomus spinal AVMs (lesions known to have similar angioarchitectural features as found in intracranial AVMs)[39], a technique similar in some ways to our technique of partial nidus electrocoagulation. And a recent pooled analysis of treatment outcomes for spinal glomus AVMs has shown that hemorrhagic risk can be significantly reduced by partial endovascular treatment[40]. In this sense, although the ultimate goal of surgical treatment is AN elimination and AVM extripation, care can be tailored according to the individual lesion, whereby a less invasive, more palliative treatment option can be employed as the initial preemptive strategy.
Radiation therapy as a primary treatment for asymptomatic brain AVMs or as an adjunctive therapy for recurrent AVMs has been advocated for treatment of lesions smaller than 3 cm in diameter and in deep or eloquent brain areas depending on size, angioarchitecture, and other factors, greater than 1- to 3-year obliteration rates range from 60% to 90%[34,41,42]. Moreover, radiosurgery for brainstem AVMs can attain up to 88% complete obliteration[43] and other reports claim complete obliteration rates in approximately two thirds of patients with a 1.7% annual hemorrhage rate for the first 3 years[44]. However, well known drawbacks include a 2.5% to 10% annual hemorrhage risk until complete obliteration, the potential for delayed hemorrhage after angiographically proven obliteration, and depending on location, there is a reported risk of clinically significant complications directly from radiosurgery for AVMs in excess of 3% to 6%, especially in cases of brainstem and other deep seated AVMs[34,43-45]. Radiosurgery for AVMs of the optic nerve and surrounding areas have been reported to cause optic neuropathy and injury to nearby neurovascular structures and consequently recommendations for maximal doses have been limited to less than 8 Gy[46]. And a study of Gamma Knife surgery for pituitary adenomas reported a 4.1% incidence of new visual dysfunction[47]. However, stereotactic radiosurgery for trigeminal neuroma presenting with trigeminal neuralgia has been shown to achieve symptomatic control without additional deficits[48]. Finally, there have been recommendations for other alternative management strategies including endovascular embolization for preoperative reduction of arterial inflow, occlusion of deep feeders, and prenidal aneurysm treatment, however, embolization in general has been associated with relatively high rates of morbidity and mortality (0% to 27%), mostly because of inadvertent embolization of feeding arteries or draining veins in normal surrounding neurovascular structures or post-embolization bleeding due to changes in flow dynamics[34]. Recently, primary embolization treatment with Onyx has shown total AVM obliteration rates of approximately 50%[49].
Major limitations of our study include a relatively brief 2.5 year follow-up in a single case report. Furthermore, we did not perform confirmatory follow-up DSA for verifying persistent patency, degree of alternative feeders, or other evidence of recurrence, however, this was a well informed decision in respect of the patient’s ongoing preference for less invasive imaging modalities such as MRI which as mentioned, has been shown to be useful for therapeutic decision making during both the preoperative and postoperative periods as well as for identification of cryptic angiographically occult AVMs. However, as in our case, follow-up MRI showed diminished AICA and nidal flow, with persistence of venous shunt flow. Indeed, mere MRI or MRA confirmation may only show relative flow reduction or stasis in flow and may not accurately represent actual residual or persistent vascular supply. In addition, clip artifact on MRI may prevent accurate assessment, while other testing such as 3D-CTA or DSA, may provide a more definitive understanding of the angioarchitecture. Standard follow-up DSA and application of additional therapy were offered to the patient, but the patient declined and ultimately application of such modalities were considered to be implemented on an as-needed basis in the event of suspected development of recurrence. We surmised that either thrombosis or diminished flow resulted in apparent radiological devascularization of the ANs and no associated clinical recurrence, however, the persistence of AVM shunt flow on follow-up MRI indicates that there is a continued risk of bleeding, although perhaps much less risk than without feeder artery and AN clipping. Furthermore, persistent external carotid artery or other feeding sources, or the potential for added changes in flow dynamics from our treatment could lead to subsequent AVM regrowth or bleeding, bleeding from the proximal basilar-AICA takeoff AN, or other de novo AN development. Currently the patient is symptom free and continues to express no interest in invasive testing or further treatment with Gamma Knife radiosurgery or other modalities.