Published online Jul 16, 2015. doi: 10.12998/wjcc.v3.i7.640
Peer-review started: August 14, 2014
First decision: September 14, 2014
Revised: April 23, 2015
Accepted: May 16, 2015
Article in press: May 18, 2015
Published online: July 16, 2015
Processing time: 351 Days and 13.7 Hours
AIM: To investigate the differentiation of human Wharton’s jelly derived mesenchymal stromal cells (WJ-MSCs) to insulin producing clusters (IPC) this study was conducted.
METHODS: The umbilical cords samples were collected from full term caesarian section mothers and the WJ-MSCS were cultured from tissue explants in High glucose-Dulbecco’s Modified Eagle Medium (H-DMEM); H-DMEM supplemented with 10% fetal bovine serum (FBS) and antibiotics. The expression of CD90, CD44, CD105, CD34 and CD133 as well as osteogenic and adipogenic differentiation of cells in appropriate medium were also evaluated. The cells were differentiated toward IPC with changing the culture medium and adding the small molecules such as nicotinic acid, epidermal growth factor, and exendin-4 during 3 wk period. The gene expression of PDX1, NGN3, Glut2, insulin was monitored by reveres transcription polymerase chain reaction method. The differentiated clusters were stained with Dithizone (DTZ) which confirms the presence of insulin granules. The insulin challenge test (low and high glucose concentration in Krebs-Ringer HEPES buffer) was also used to evaluate the functional properties of differentiated clusters.
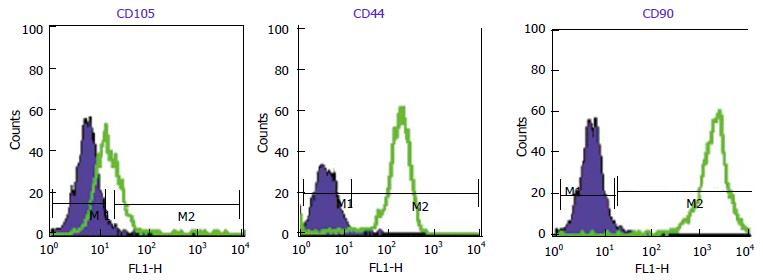
RESULTS: WJ-MSCS were positive for mesenchymal surface markers (CD90, CD44, CD105), and negative for CD34 and CD133. The accumulation of lipid vacuoles and deposition of calcium mineral in cells were considered as adipogenic and osteogenic potential of WJ-MSCS. The cells also expressed the transcriptional factors such as Nanog and OCT4. During this three step differentiation, the WJ-MSCS morphology was gradually changed from spindle shaped cells in to epithelioid cells and eventually to three dimensional clusters. The clusters expressed PDX1, NGN3, Glut2, and insulin. The cells became bright red color when stained with DTZ and the insulin secretion was also confirmed. In glucose challenge test a significant increase in insulin secretion from 0.91 ± 0.04 μIu/mL (2.8 mmol/L glucose) to to 8.34 ± 0.45 μIu/mL (16.7 mmol/L glucose) was recorded (P < 0.05). The insulin secretion of undifferentiated WJ-MSCS was not changed in this challenge test.
CONCLUSION: WJ-MSCs have the ability to differentiate in to islet-like cells in vitro. However, this process needs further optimization in order to generate efficient and functional IPCs.
Core tip: Diabetes is a major chronic metabolic disorder in the world. Mesenchymal stromal cells (MSCs) has the ability to differentiate in to functional insulin producing cells.In this study, human Wharton’s jelly derived MSCs (The clusters expressed PDX1, NGN3, Glut2, and insulin. The cells became red color when stained with DTZ and the insulin secretion was confirmed Wharton’s jelly derived MSCs were differentiated to insulin producing clusters (IPCs). More efficient differentiation protocoles for generation of functional IPCs will be a potential new source for cell transplantation in diabetic patients.
-
Citation: Nekoei SM, Azarpira N, Sadeghi L, Kamalifar S.
In vitro differentiation of human umbilical cord Wharton’s jelly mesenchymal stromal cells to insulin producing clusters. World J Clin Cases 2015; 3(7): 640-649 - URL: https://www.wjgnet.com/2307-8960/full/v3/i7/640.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i7.640
Diabetes mellitus is characterized by an absolute or relative lack of blood insulin and associated the impaired metabolism of carbohydrates, fats and proteins. Recent study reported that 382 million people had diabetes in the world; and by 2035, this number is expected to be increased to 592 million[1]. Pancreas or islet cell transplantation is considered as an effective therapy for diabetic patients[2]. However, the limited number of cadaveric donors and immunological rejection are two major obstacles to achieve effective long term results[3].
Human embryonic stem cell (ESC) is a good promising source for treating diabetes[4]. However, ethical concerns about the use of human embryos and the risk of tumourigenicity are problems regarding the use of these cells for clinical use[4]. Other studies have made efforts to differentiate pancreatic beta cells from other sources such as induced pleuripotent stem cells (IPSs)[5,6], bone marrow-derived mesenchymal stromal cells[7,8], or umbilical cord blood cells[9].
Mesenchymal stromal cells (MSCs) are isolated from the Wharton’s jelly or umbilical cord, are easily obtained and processed compared to ESCs and bone marrow derived MSCs[10]. The cells have differentiated into adipogenic, chondrogenic, and osteogenic lineage and also have expressed the CD105, CD44 and CD90 and negative for hematopoietic and vascular markers such as: CD33, CD56, CD31, CD34, CD45[10]. Wharton’s jelly derived MSCs (WJ-MSCs), have high proliferation capacity and do not induce teratomas after transplantation[10].
The potential of these postnatal stem cells to differentiate toward insulin-producing cells has been evaluated previously. However, the current protocols are not optimized for efficient transdifferentiation. Here, we describe the three step modified protocol for direct differentiation of WJ-MSCs into insulin producing clusters (IPC). These clusters produce insulin in response to different glucose concentration.
Human umbilical cord samples were aseptically collected from full-term delivery by cesarean section at the Hafez Hospital affiliated to Shiraz University of Medical Sciences. Informed consent was received from mothers and the study design was approved by ethical committee of our university.
Umbilical cord Wharton’s jelly was processed within 2 h after aseptic collection and cut into pieces of 0.5-1 mm2. These pieces were placed in 10 cm plates and cultured in High glucose-Dulbecco’s Modified Eagle Medium; H-DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin 100 U/mL, streptomycin 100 μg/mL. The plates were placed in an incubator with saturated humidity at 37 °C containing 5% CO2. The medium was changed every three days; the cell migrated from the margins of explants. After reaching 70%-80% confluence, the adherent cells were harvested with 0.05% trypsin-Ethylene diamine tetra acetic acid (EDTA), (Gibco, Germany) and the cell suspension was used for subsequent experiments. The presence of transcription factors that regulate maintenance of pluripotent state in ESC (OCT4, Nanog) were studied by reveres transcription polymerase chain reaction (RT-PCR).
The WJ-MSCs was stained with monoclonal antibodies: CD90, CD44, CD105, CD34 and CD133 (BioLegend, San Diego, Calif., United States) and analyzed using the FACS Calibur flow cytometer (Becton Dickinson, NJ, United States).
To investigate their capacity for mesodermal differentiation adipogenic and osteogenic differentiation was carried out by culturing cells with appropriate differentiation medium. For adipocytes differentiation, the WJ-MSCs were cultured in L-DMEM supplemented with 10% FBS, 10 nmol/L dexamethasone, 0.5 μg/mL insulin, 2 mmol/L glutamine for 15 d. Then the cells were fixed with 0.4% paraformaldehyde (PFA) and stained with oil-red-O (Sigma). For osteoblastic differentiation, the WJ-MSCs were cultured L-DMEM supplemented with 10% FBS, 10 nmol/L dexamethasone (Sigma), 10 mmol/L b-glycerol phosphate (Sigma) and 50 mg/mL ascorbic acid-2 phosphate (Sigma). After fixation, the differentiated cells were stained with alizarin red stain and calcium deposition was confirmed.
The cultured WJ-MSCs from 4th passage with 80% to 90% confluency were induced to differentiate into IPC in three separate stages.
In first sage, the MSC monolayer was treated for 24 h with high glucose Dulbecco modified Eagle medium-F12 (DMEM-F12) supplemented with10% FBS, 10-6 mol/L of retinoic acid (RA; Sigma-Aldrich) and 1% antibiotic; then the medium was switched to DMEM-F12 containing only 10% FBS for 2 d.
In second stage, the cells were detached with 0. 5% trypsin-EDTA and seeded in 0.1% gelatin (Sigma-Aldrich) coated plates. The medium was switched to DMEM-F12, supplemented with 10% FBS, 10 mmol/L nicotinic acid (Sigma-Aldrich), and 20 ng/mL epidermal growth factor (EGF, Sigma-Aldrich) for one week.
In third stage, in order to mature the insulin-producing cells, the DMEM-F12 medium was supplemented with 10% FBS and exendin-4 (Sigma-Aldrich) for one week. Glucagon-Like Peptide-1 or Exendin-4 is an insulin secretagogue, with glucoregulatory effects.
The formation of islet like clusters was daily monitored and the DTZ staining confirmed the presence of insulin granules. The genes expression of pancreatic endocrine cell development was monitored in the end of each step. The cells which were cultured in L-DMEM containing only 10% FBS was considered as a control group.
The total RNA was extracted from WJ-MSCs s before and after third stage of differentiation by using Mini-RNease RNA extraction kit (Cinnagen, Iran). The cDNA was synthesized using MMULV reverse transcriptase (Cinnagen, Iran). The reaction condition for cDNA synthesis was: pre-heating at 42 °C for 90 min; followed by annealing at 85 °C for 5 s. The synthesized cDNA was used to examine the expression of pancreatic specific transcription factors as listed in Table 1. Amplification used a fluorescence-quantitative PCR instrument (ABI Prism7500, step one plus) by using SYBER Green (TAKARA, Japan) following the manufacturer’s instructions. Amplification was carried out as follows: denaturing at 95 °C for 5 min; followed by 40 cycle denaturation at 95 °C for 1 min; annealing for 1 min. The final stage was run to generate a melting curve for verification of amplification product specificity. Beta actin gene was used as a control. The products were also visualized by gel electrophoresis.
| Names | Forward (F) 5´-3´ | Reverse(R) 5´-3´ |
| Insulin | GCAGCCTTTGTGAACCAACA | TTCCCCGCACACTAGGTAGAGA |
| PDX1 | GGATGAAGTCTACCAAAGCTCACGC | CCAGATCTTGATGTGTCTCTCGGTC |
| NGN3 | CAATCGAATGCACAACCTCA | GGGAGACTGGGGAGTAGAGG |
| GLUT2 | AGGACTTCTGTGGACCTTATGTG | GTTCATGTCAAAAAGCAGGG |
| NANOG | CAGAAGGCCTCAGCACCTAC | ATTGTTCCAGGTCTGGTTGC |
| Oct-4 | CAGTGCCCGAAACCCACAC | GGAGACCCAGCAGCCTCAAA |
| β-actin | GATCGGCGGCTCCATCCTG | GACTCGTCATACTCCTGCTTGC |
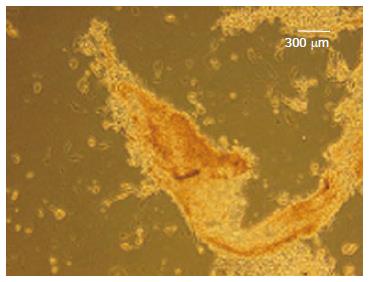
Diphenylthiocarbazone, Dithizone (DTZ), (Sigma, United States) is a zinc-chelating substance which is used for identification of insulin granules in pancreatic beta-cells as bright crimson red. The Acinar cells remain unstained.
In order to evaluation insulin production in IPC at the end of 3rd week, the differentiated clusters were examined.
The clusters, were rinsed twice in Krebs-Ringer HEPES (KRH) buffer (125 mmol/L NaCl, 4.74 mmol/L KCL, 1 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 5 mmol/L NaHCO3, 25 mmol/L HEPES, 0.1 mmol/L 0.1%BSA, pH = 7.4) containing 2.8 mmol/L glucose (Low glucose concentration) (Sigma-Aldrich, United States).
After 1 h incubation, the medium was collected for insulin assay and the cells were stimulated with KRH buffer containing 16.7 mmol/L glucose for 1 h (High glucose concentration). The insulin level was determined by Enzyme linked Immunoassay kit and the results were compared with undifferentiated MSC cells as control group.
The data were presented as means ± SD. Each experiment was repeated 3 times. Data was assessed by one-way ANOVA followed by Tukey’s test for pair wise comparison. P < 0.05 was considered as statistically significant. The statistical analysis was performed using Graph Pad Prism 5 software.
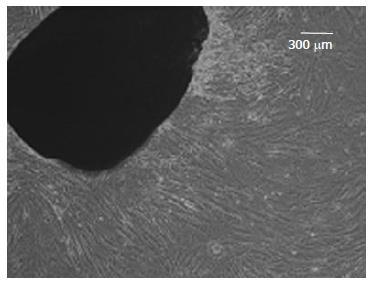
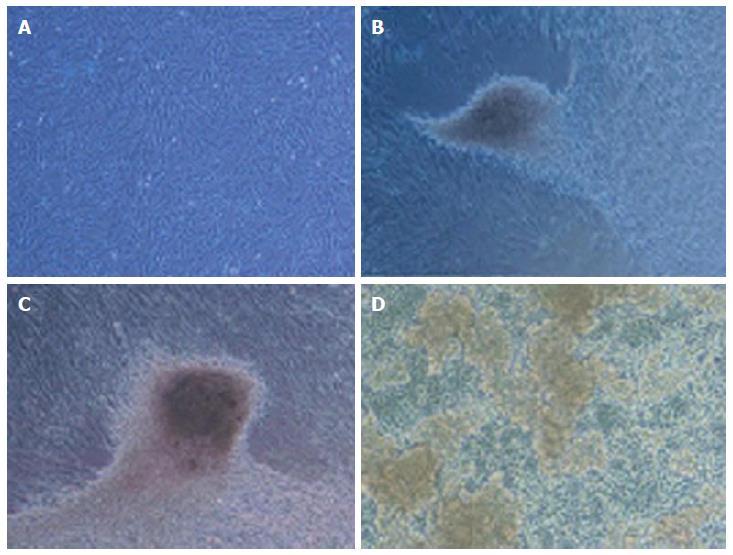
WJ-MSCS formed a homogenous monolayer of adherent spindle shaped cells (Figure 1). Flow cytometry analyses showed that the WJ-MSCS were positive for mesenchymal markers (CD90, CD44, CD105), and negative for CD34 and CD133 (Figure 2). The ESCs transcriptional factors such as Nanog and OCT4, also expressed.
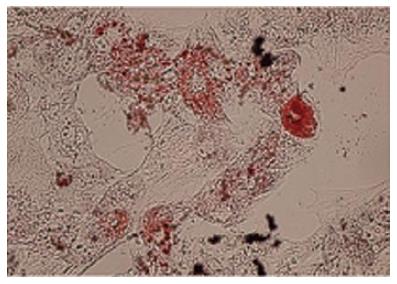
The accumulation of lipid vacuoles (Stained red color in Oil Red O) in cells was considered as adipogenic differentiation. Deposition of calcium minerals (stained as orange red color with Alizarin Red) was also considered as osteogenic potential of these cells (Figures 3 and 4).
In passage 4 (P4), the WJ-MSCS were step wise differentiated toward insulin producing cells.
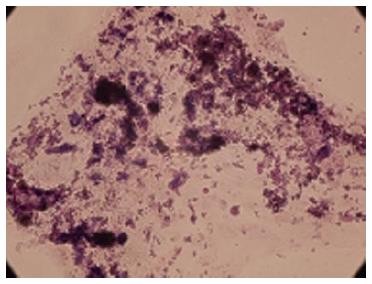
In stage 1, the cells have shown spindle-shaped morphology (Figure 5A). Cell morphology was gradually changed into round epithelioid cells and by addition of beta cell maturation factor such as nicotinamide in stage 3, three dimensional clusters were formed (Figure 5B-D). These clusters were stained red color with DTZ (Figure 6).
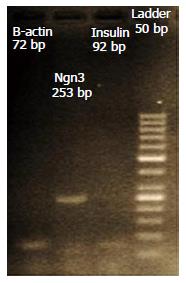
After differentiation, mRNA expression of pancreatic development transcription factors and beta cell specific gene such as PDX1, NGN3, Glut2 and insulin were detected. Expressions of the mentioned genes led to in vitro production of functional IPCs. Results represent three separate experiments (Figure 7A and B).
In respect of insulin secretion, a significant increase in insulin secretion from 0.91 ± 0.04 μIu/mL (2.8 mmol/L glucose) to to 8.34 ± 0.45 μIu/mL (16.7 mmol/L glucose) was recorded (P < 0.05). The insulin secretion was detected in undifferentiated WJ-MSCS (0.3 ± 0.03 μIu/mL) which was not changed in glucose challenge test.
Type 1 diabetes mellitus is caused by an autoimmune destruction of pancreatic beta cells. Although the insulin therapy remains the routine treatment for diabetes, but whole pancrease organ transplant or transplantation of pancreatic islets of Langerhans provides a cure for this disorder[2,3]. Various types of stem cells such as ESCs, induced pleuripotent stem cells (IPs) and mesenchymal stromal cells have been differentiated into IPCs by genetic modification and/or modification in culture conditions[7,8,11,12]. Using of ESCs has several limitations such as ethical problem, immune rejection, and risk of tumorigenesis.
To overcome these problems, human IPS is ideal for personalized therapy. However, epigenetic changes, and chromosomal instability during reprogramming remain the obstacle[6] .
Generation of IPCs from MSCs from a variety of tissues such as bone marrow, umbilical cord, and adipose tissue represents an alternative therapy. MSCs can also be used as a cellular vehicle for the expression of human insulin[6,13-15].
The MSC numbers in bone marrow and umbilical cord blood are low and require multiple ex vivo expansion. Extra-embryonic tissue such as fetal membrane and umbilical cord Wharton’s jelly has the stemness phenotype, immunoprivileged properties, and faster proliferation than adult MSCs and is considered as unlimited source for tissue engineering and regenerative medicine[13]. The WJ-MSCs express HLA-G6 isoform, the unique ability which is important in immune modulation. Therefore, these cells are particularly suitable for cell based therapy. These tissues are normally discarded after birth and using them is not associated with ethical problem[16,17].
In this study, WJ-MSCs were isolated and their differentiation into adipocytes, and osteocytes confirmed their multilineage potentials. The retinoic acid we used to start the differentiation process. RA is an essential molecule for dorsal pancreas development in mouse. The effects of RA are achieved through RA binding and activation of retinoic acid receptors such as RARα, RARβ, and RARγ. Over expression of RARβ was detected in early stage of pancreases differentiation and absence of RARβ impaired the terminal differentiation of α and β-cells[18,19]. In vitamin-A deficiency, pancreatic islet function was Impaired. RA directly induces Pdx1 expression in ESCs and Pdx1 is an important transcription factor in the early development of pancreatic progenitors and bud expansion[20,21]. The retinoic acid response element is located at upstream of the transcription start site of Pdx1. Retinoie Acid Receptor (RARβ) expression is depended on epigenetic regulation[18,19]. Hyper methylation of the RARβ2 promoter was reported in pancreatic cancer, and diabetes. Therefore, it is postulated that the epigenetic silencing of RARβ, combined with vitamin A deficiency, may play a role in pathogenesis of diabetes[18,19]. Glucose is considered as a growth factor for β-cells replication both in vitro and in vivo[22]. Subsequently, using of EGF was associated with proinsulin biosynthesis and 3H-thymidine incorporation in experimental model[23,24]. Nicotinamide was used during the second stage of differentiation. Various published protocols reported this substance as an effective inducer in pancreatic differentiation. Nicotinamide preserve islet viability through poly-ADP-ribose polymerase[25].
Glucagon-like peptide 1 (GLP-1) and its long acting mimetic exendin-4 are usually used for treatment of type 2 diabetes. Exendin 4 simultaneously stimulates beta cell secretory capacity as well as maintains insulin stores by translational control of proinsulin biosynthesis. Exendin-4 can also stimulate b-cell replication in human islets from young donors[26-28].
In our study, at the end of in vitro differentiation protocols, pancreatic endocrine genes and insulin were expressed. However, the insulin secretion after glucose challenge test was very low. As mentioned previously, incomplete beta cell phenotype and consequently poor insulin release in response to glucose challenge test might explain this problem[29,30].
In the literature, various sources of mesenchymal stem were differentiated into insulin secreting cells with different efficacy[31-43] (Table 2). However, the insulin secretion capacity of the cells was variable. The insulin challenge test was done with different protocols; the concentration of insulin was measured with different assays and reference range. Therefore, the comparison of data was not easily performed.
| Stem cell sources | Differentiation protocol | Efficiency(generation of insulin producing cell) | Ref. |
| Placenta-derived Mesenchymal stem cells | (α-MEM + 1% BSA + 1 × ITS + 0.3 mmol/L taurine) 3 d (α-MEM + 1.5% BSA + 1 × ITS + 3 mmol/L taurine + GLP-1 + nicotinamide) 7 d (α-MEM + 1.5% BSA + 1 × ITS + 3 mmol/L taurine + GLP-1 + nicotinamide) 10 d | 65%-70% ILCs (represents 20%-25% beta-cells per islet) | Kadam et al[31] |
| Human embryonic stem cells | (DMEM-F12, 20% SR, 2 mmol/L GlutaMAX, 1% NEAA and 0.1 mmol β-mercaptoethanol) 7 d (DMEM-F12, 1% ITS, 2 mmol/L GlutaMAX, 5 μg/mL Fibronectin) 7 d (DMEM-F12, 1% N2, 2% B27, 2 mmol/L GlutaMAX, 10 ng/mL bFGF) 7 d (DMEM-F12, 1% N2, 2% B27, 2 mmol/L GlutaMAX, and 10 mmol/L nicotinamide) for 7-9 d | 61.7% ± 9.5% insulin positive cells | Wei et al[32] |
| Human embryonic stem cells | (DMEM-F12, 100 ng/mL activin A, 1 μmol/L wortmannin, 1% N2, 1% B27) 4 d (IMDM/F12, 2 μmol/L retinoic acid, 20 ng/mL FGF7, 50 ng/mL Noggin, 0.25 μmol/L KAAD-cyclopamine, 1% B27) 4 d (DMEM, 50 ng/mL EGF, 1% ITS, 1% N2) 5 d (DMEM-F12, 1% ITS, 10 ng/mL bFGF, 10 mM nicotinamide, 50 ng/mL exendin-4) 7-9 d | 41.6% ± 11.8% insulin positive cells | Wei et al[32] |
| hESC lines YT1 and YT2 | (RPMI1640, 0.2% FBS, 0.56 N2, 0.56 B27, 100 ng/mL activin A, 1 mmol/L wortmannin) 4 d (RPMI1640, 0.5% FBS, 0.5% ITS, 0.56 B27, 2 mM retinoic acid, 20 ng/mL FGF-7, 50 ng/mL Noggin) 4 d (DMEM, 0.5% FBS, 1% ITS, 16 N2, 50 ng/mL EGF) 5 d (DMEM/F12, 1% ITS, 10 ng/mL bFGF, 10 mmol/L nicotinamide, 50 ng/mL exendin-4, 10 ng/mL BMP4) until maturation | 17.1% insulin positive cells | Hua et al[33] |
| Human embryonic stem cells | (MCDB-LG, 100 ng/mL GDF8, 2.5 mmol/L MCX-928, 100 ng/mL) 1 d (MCDB-LG, 100 ng/mL GDF8) 2-4 d (MCDB-LG, 50 ng/mL FGF7) 2 d (MCDB-HG, 50 ng/mL FGF7, 20 ng/mL ActivinA, 0.25 μmol/L SANT-1, 2 μmol/L Retinoic Acid, 200 nmol/L LDN193189) 4 d (MCDB-HG, 0.25 μmol/L SANT-1, 200 nmol/L LDN193189, 500 nmol/L TBP, 100 nmol/L CYP26A inhibitor) 3 d (MCDB-HG, 200 nmol/L LDN193189, 1 μmol/L ALK5i, 100 nmol/L CYP26A inhibitor) 3 d (MCDB-HG, 200 nmol/L LDN193189, 1 μmol/L ALK5i) 3 d (MCDB-HG, 200 nmol/L LDN193189, 1 μmol/L ALK5i, 100 nmol/L VitaminA) 7-14 d | 6%-10% insulin positive cells | Bruin et al[34] |
| Human embryonic stem cells | (EB formation) 2 d (DMEM-F12, KSR, ActivinA, Retinoic acid) 6 d (DMEM-F12, KSR, bFGF, Noggin) 12 d (DMEM-F12, N2, B27, Laminin, bFGF, Nicotinamid, GLP-1) 12 d (CMRL1066, Nicotinamid, ITS, Zn2SO4, Glutamax, HEPES, KSR, GLP-1, Exendin1, HGF) 10 d | 24.5% insulin producing cell | Bose et al[35] |
| Bone marrow mesenchymal stem cells | (Human BMSCs were transfected with adenovirus carrying PDX1 or VEGF) 2 d | 50% of cells was differentiated to beta cell | Milanesi et al[36] |
| Human bone marrow-derived stem cells | (RPMI 1640, 5.5 mmol/L glucose, 5% FCS, 10 mmol/L nicotinamide, 10 nmol/L exendin 4) 5 d | 20% insulin producing cell | Tang et al[37] |
| Human bone marrow-derived mesenchymal stem cells | (DMEM, 0.5 mmol/L β-mercaptoethanol) 2 d ( DMEM, 1% non-essential amino acids, 20 ng/mL bFGF, 20 ng/mL EGF, 2% B27, 2 mmol/L L-glutamine) 8 d (DMEM, 10 ng/mL betacellulin, 10 ng/mL activin-A, 2% B27, 10 mmol/L nicotinamide) 8 d | 5% insulin producing cell | Gabr et al[38] |
| Human labia minor dermis-derived fibroblasts | (DMEM/F12, ITS) 7 d (DMEM, 10% FBS, 100 U/mL penicillin/streptomycin, ITS, 10 mmol/L nicotinamide) 7 d | 50% insulin positive cell 2 × 106 cells generated around 400–600 ICAs | Kim et al[39] |
| Human adipose tissue derived adult stem cells | (DMEM/F12, 17.5 mmol/L glucose, 1% BSA, 1 × ITS, 4 nmol/L activin A, 1 mmol/L sodium butyrate, 50 mmol/L 2-mercaptoethanol, 2 ng/mL FGF) 2 d (DMEM/F12, 17.5 mmol/L glucose, 1% BSA, ITS, 0.3 mmol/L Taurine) 2 d (DMEM/F12, 17.5 mmol/L glucose, 1.5% BSA, ITS, 3 mmol/L Taurine, 100 nmol/L GLP-1, 1 mmol/L nicotinamide and 1× non-essential amino acids) 12-14 d | Chandra et al[40] | |
| Human Adult Fibroblast-Like Limbal Stem Cells | (RPMI-1640, 100 ng/mL Activin A) 2-3 d (RPMI-1640, 2% FBS, 50 ng/mL bFGF) 3-4 d (DMEM, 10% FBS, 1% B27, 2% N2, 1 mmol/L nicotinamide) 3-4 d (DMEM, 10% FBS, 1% B27, 2% N2, 1 mmol/L nicotinamide, 50 ng/mL exendin-4) 3-4 d | 70% to 77% insulin producing cell | Criscimanna et al[41] |
| (RPMI-1640, 100 ng/mL Activin A) 2-3 d (RPMI-1640, 2% FBS, 50 ng/mL FGF 10 μmol/L) 3-4 d (DMEM, 1% B27, 50 ng/mL FGF 10.2 μmol/L retinoic acid) 3-4 d (DMEM, 1% B27, 50 ng/mL exendin-4) 3-4 d | |||
| Dental pulp stem cells | (DMEM-KO, 1% BSA, 1x ITS, 4 nmol/L activin A, 1 mM sodium butyrate, 50 μmol/L 2-mercaptoethanol) 2 d (DMEM-KO, 1% BSA, ITS, 0.3 mmol/L taurine) 2 d (DMEM-KO, 1.5% BSA, ITS, 3 mmol/L taurine, 100 nmol/L GLP-1, 1 mmol/L nicotinamide, 1x non-essential amino acids) 5 d | 2 × 106 (Number of cells generated from around 156 ± 23 ILCs) | Govindasamy et al[42] |
| Stem cells from human exfoliated deciduous teeth (SHED) | (KO-DMEM, 1% BSA, 1 × ITS, 4 nmol/L activinA, 1 mmol/L sodium butyrate) 2 d (KO-DMEM, 1% BSA, ITS, 0.3 mmol/L taurine) 1 d (KO-DMEM, 1.5% BSA, ITS, 3 mmol/L taurine, 100 nmol/L glucagon-like peptide-1, 1 mmol/L nicotinamide) 7 d | 231 ± 21 number of generated ILCs from initial cell seeding density 2 × 106 | Kanafi et al[43] |
| Dental pulp stem cells | 112 ± 2 number of generated ILCs from initial cell seeding density 2 × 106 | Kanafi et al[43] |
In our experience, differentiation of WJ-MSCs to form IPCs needs further optimization for clinical practice. To overcome this problem, addition of growth factors, extracellular matrix and/or culturing the cells in three dimensional environments are suggestive.
The present article was adopted from Masumeh Nekoui MsC thesis in Embryology and development. The authors are also grateful to Mrs Elaheh Esfandiari and Mrs Masoumeh Darai for their assistance in cell culture and PCR.
Different types of stem cells such as embryonic stem cells (ESCs), induced pleuripotent stem cells and mesenchymal stromal cells have been differentiated into insulin producing cells by modification in cell culture conditions and addition of small molecules. Umbilical cord Wharton’s jelly derived stromal cells has the stemness phenotype, with faster proliferation than adult mesenchymal stromal cells (MSCs) and is considered as a good source for generation of insulin producing cells.
In this research, insulin producing clusters (IPC) was differentiated from Wharton’s jelly MSCs (WJ-MSCs) without any genetic manipulation.
The IPCs was differentiated from WJ-MSCs by changing the cell culture medium and growth factors without genetic manipulation. However, the insulin release in response to glucose challenge test was not sufficient. Similar studies used various sources of mesenchymal stem and differentiated into insulin secreting cells with different efficacy and variable insulin secretion capacity. The insulin challenge test was done with different protocols; the concentration of insulin was measured with different assays and reference range. Therefore, the comparison of data was not easily performed.
Differentiation of WJ-MSCs to form IPCs needs further optimization for clinical practice.
The authors have performed a good study, the manuscript is interesting.
P- Reviewer: Fabozzi M, Pan HC S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2955] [Article Influence: 268.6] [Reference Citation Analysis (1)] |
| 2. | Azarpira N, Aghdai MH, Nikeghbalian S, Geramizadeh B, Darai M, Esfandiari E, Bahador A, Kazemi K, Al-Abdullah IH, Malek-Hosseini SA. Human islet cell isolation: the initial step in an islet transplanting program in Shiraz, Southern Iran. Exp Clin Transplant. 2014;12:139-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 324] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [PubMed] |
| 5. | Shaer A, Azarpira N, Karimi MH. Differentiation of human induced pluripotent stem cells into insulin-like cell clusters with miR-186 and miR-375 by using chemical transfection. Appl Biochem Biotechnol. 2014;174:242-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Shaer A, Azarpira N, Vahdati A, Karimi MH, Shariati M. Differentiation of human-induced pluripotent stem cells into insulin-producing clusters. Exp Clin Transplant. 2015;13:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Gabr MM, Zakaria MM, Refaie AF, Khater SM, Ashamallah SA, Ismail AM, El-Badri N, Ghoneim MA. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Jafarian A, Taghikhani M, Abroun S, Pourpak Z, Allahverdi A, Soleimani M. Generation of high-yield insulin producing cells from human bone marrow mesenchymal stem cells. Mol Biol Rep. 2014;41:4783-4794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Bhonde RR, Sheshadri P, Sharma S, Kumar A. Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int J Biochem Cell Biol. 2014;46:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F, La Rocca G. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7:342-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Sun NZ, Ji HS. In vitro differentiation of human placenta-derived adherent cells into insulin-producing cells. J Int Med Res. 2009;37:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Dou Z. Under a nonadherent state, bone marrow mesenchymal stem cells can be efficiently induced into functional islet-like cell clusters to normalize hyperglycemia in mice: a control study. Stem Cell Res Ther. 2014;5:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Paek HJ, Kim C, Williams SK. Adipose stem cell-based regenerative medicine for reversal of diabetic hyperglycemia. World J Diabetes. 2014;5:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 15. | Yuan H, Li J, Xin N, Zhao Z, Qin G. Expression of Pdx1 mediates differentiation from mesenchymal stem cells into insulin-producing cells. Mol Biol Rep. 2010;37:4023-4031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 916] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 17. | Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s Jelly-Derived Mesenchymal Stem Cells: Phenotypic Characterization and Optimizing Their Therapeutic Potential for Clinical Applications. Int J Mol Sci. 2013;14:11692-11712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Mühlbauer E, Bazwinsky-Wutschke I, Wolgast S, Labucay K, Peschke E. Differential and day-time dependent expression of nuclear receptors RORα, RORβ, RORγ and RXRα in the rodent pancreas and islet. Mol Cell Endocrinol. 2013;365:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Pérez RJ, Benoit YD, Gudas LJ. Deletion of retinoic acid receptor β (RARβ) impairs pancreatic endocrine differentiation. Exp Cell Res. 2013;319:2196-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Oström M, Loffler KA, Edfalk S, Selander L, Dahl U, Ricordi C, Jeon J, Correa-Medina M, Diez J, Edlund H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS One. 2008;3:e2841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dollé P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Cras-Méneur C, Elghazi L, Czernichow P, Scharfmann R. Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes. 2001;50:1571-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Chatterjee AK, Sieradzki J, Schatz H. Epidermal growth factor stimulates (pro-)insulin biosynthesis and 3H-thymidine incorporation in isolated pancreatic rat islets. Horm Metab Res. 1986;18:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kolb H, Burkart V. Nicotinamide in type 1 diabetes. Mechanism of action revisited. Diabetes Care. 1999;22 Suppl 2:B16-B20. [PubMed] |
| 26. | Tourrel C, Bailbé D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50:1562-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Alarcon C, Wicksteed B, Rhodes CJ. Exendin 4 controls insulin production in rat islet beta cells predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia. 2006;49:2920-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wu L, Olverling A, Huang Z, Jansson L, Chao H, Gao X, Sjöholm Å. GLP-1, exendin-4 and C-peptide regulate pancreatic islet microcirculation, insulin secretion and glucose tolerance in rats. Clin Sci (Lond). 2012;122:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837-2844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Boyd AS, Wu DC, Higashi Y, Wood KJ. A comparison of protocols used to generate insulin-producing cell clusters from mouse embryonic stem cells. Stem Cells. 2008;26:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Wei R, Yang J, Hou W, Liu G, Gao M, Zhang L, Wang H, Mao G, Gao H, Chen G. Insulin-producing cells derived from human embryonic stem cells: comparison of definitive endoderm- and nestin-positive progenitor-based differentiation strategies. PLoS One. 2013;8:e72513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Hua XF, Wang YW, Tang YX, Yu SQ, Jin SH, Meng XM, Li HF, Liu FJ, Sun Q, Wang HY. Pancreatic insulin-producing cells differentiated from human embryonic stem cells correct hyperglycemia in SCID/NOD mice, an animal model of diabetes. PLoS One. 2014;9:e102198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Bruin JE, Erener S, Vela J, Hu X, Johnson JD, Kurata HT, Lynn FC, Piret JM, Asadi A, Rezania A. Characterization of polyhormonal insulin-producing cells derived in vitro from human embryonic stem cells. Stem Cell Res. 2014;12:194-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Bose B, Shenoy SP, Konda S, Wangikar P. Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes. Cell Biol Int. 2012;36:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Milanesi A, Lee JW, Li Z, Da Sacco S, Villani V, Cervantes V, Perin L, Yu JS. β-Cell regeneration mediated by human bone marrow mesenchymal stem cells. PLoS One. 2012;7:e42177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Tang DQ, Wang Q, Burkhardt BR, Litherland SA, Atkinson MA, Yang LJ. In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am J Stem Cells. 2012;1:114-127. [PubMed] |
| 38. | Gabr MM, Zakaria MM, Refaie AF, Ismail AM, Abou-El-Mahasen MA, Ashamallah SA, Khater SM, El-Halawani SM, Ibrahim RY, Uin GS. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2013;22:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Kim B, Yoon BS, Moon JH, Kim J, Jun EK, Lee JH, Kim JS, Baik CS, Kim A, Whang KY. Differentiation of human labia minora dermis-derived fibroblasts into insulin-producing cells. Exp Mol Med. 2012;44:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Chandra V, Swetha G, Muthyala S, Jaiswal AK, Bellare JR, Nair PD, Bhonde RR. Islet-like cell aggregates generated from human adipose tissue derived stem cells ameliorate experimental diabetes in mice. PLoS One. 2011;6:e20615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Criscimanna A, Zito G, Taddeo A, Richiusa P, Pitrone M, Morreale D, Lodato G, Pizzolanti G, Citarrella R, Galluzzo A. In vitro generation of pancreatic endocrine cells from human adult fibroblast-like limbal stem cells. Cell Transplant. 2012;21:73-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Govindasamy V, Ronald VS, Abdullah AN, Nathan KR, Ab Aziz ZA, Abdullah M, Musa S, Kasim NH, Bhonde RR. Differentiation of dental pulp stem cells into islet-like aggregates. J Dent Res. 2011;90:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Kanafi MM, Rajeshwari YB, Gupta S, Dadheech N, Nair PD, Gupta PK, Bhonde RR. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy. 2013;15:1228-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |