INTRODUCTION
Multiple sclerosis (MS) is a chronic immunologic disease in which inflammation, demyelination and axonal damage are the main pathologic features. The exact etiology of MS is unknown, however genetic susceptibility may be contributed[1]. MS has a prevalence of more than 30 cases per 100000 people[2,3]. Typically, the majority of patients with MS (approximately 70%) are young adults with an age range between 20-40 years particularly females (75%), while the remaining 10% and 30% of cases occur before the age of 20 years and after the age of 40 years, respectively[3]. MS has often nonspecific initial symptoms; however, typically, MS is presented by sensory, motor and visual dysfunctions. MS is usually diagnosed by demonstrating evidence of clinical and/or radiographic dissemination of the disease in time and space[4,5]. The typical MS plaques appear in magnetic resonance imaging of the brain (MRI) as multiple periventricular homogenous ovoid lesions ranged in size from 3 to 16 mm, often oriented perpendicular to the long axis of the ventricular system and have no mass effect. Other locations include the optic nerves, corpus callosum, centrum semiovale, pons, cerebellar peduncles or hemispheres, brainstem and spinal cord[5]. Nearly 85% of cases with MS develop relapsing-remitting MS (RRMS) course in which relapses usually occur on average once every other year. Nearly 50%-90% of cases with RRMS develop secondary progressive MS within 10-25 years, 10% develop primary progressive MS and 5% develop acute attacks on top of steadily progressive neurologic decline[6]. Pharmacologic treatment of MS include immunosuppressants and immunomodulators[7].
Some cases of MS may pose a diagnostic difficulty due to atypical clinical and neuroimaging manifestations which mimic other fulminant central nervous system (CNS) conditions as inflammatory/infective conditions and intracranial neoplastic and non-neoplastic space occupying lesions (SOLs).
This paper describes an adult woman who presented for the first time with mental confusion and rapid deterioration in different cognitive functions. She also had atypical imaging features.
CASE REPORT
At December 2009, a 25-year right handed woman was admitted to our department with a one month history of mental confusion and rapid cognitive deterioration without previous history of systemic infection or a stress-related factor. Her family reported that the patient was unable to do her daily home duties, neglected care for herself and her children and looked blind and had poverty of speech. She was referred by a neurologist who reported abnormal computed tomography (CT) of the brain with large multifocal hypodense lesions (Figure 1). On neurological evaluation upon admission, she looked apathetic, had mixed dysphasia and had marked diminution of visual acuity (hand movement) with bilateral dilated pupils which were less reactive to light and bilateral temporal pallor of the optic discs. She had normal motor and peripheral sensory examination and bilateral flexor planter responses. She had bilateral prolonged P100 component of the visual evoked potentials (VEPs) indicating demyelinating optic neuropathy (Figure 2). Conventional magnetic resonance imaging of the brain (MRI-brain) showed bilateral multiple subcortical superficial and deep white-matter large (≥ 3 cm) lesions in the frontal, parietal, temporal and occipital lobes which were hypointense in T1-weighted and hyperintense in T2-weighted and fluid-attenuated inversion recovery (FLAIR) images with minimal perifocal edema and mass effect in spite of large sizes and bilaterally of the lesions. They demonstrated incomplete or patchy contrast enhancement with gadolinium (Figure 3). Spinal MRI was normal. The patient had normal routine laboratory blood testing which included complete blood count, blood sugar, serum electrolytes, liver and kidney function tests, erythrocytic sedimentation rate, rheumatoid factor, antinuclear antibodies and anti-double stranded DNA (anti-ds DNA) antibodies. The patient was given a diagnosis of tumefactive MS. After admission, she received 1 g daily intravenous methylprednisolone for 5 d. Oral prednisolone in a dose of 80 mg twice daily was given for 1 mo. As no clinical improvement was observed, another dose of pulse steroid therapy was given which was also followed by 80 mg twice daily oral prednisolone. Near the end of the 3rd month of treatment, improvement started with rapid regression of patients symptoms in the form of improvement of cognition and vision, after which the patients was discharged from the hospital (March 2010). Clinical and MRI follow ups were done every 3 mo. We observed lack of significant MRI improvement in spite of the clinical improvement (Mini Mental State Examination = 30/30) after 6 mo from the onset of the condition (Figure 4). The patient was informed that she is in need for a disease modifying therapy to optimize therapy and prevent relapses (as IFNβ-1a/b, glatiramer acetate, mitoxantrone and natalizumab which are available in our country) but she refused due to the low socioeconomic status and she had no insurance to cover the cost of such expensive course of treatment. Significant reduction of the sizes of the MRI lesions was observed after one year of onset of the condition (Figure 5). Neither clinical relapse nor new MRI lesions were observed throughout the following 4 years of follow up.
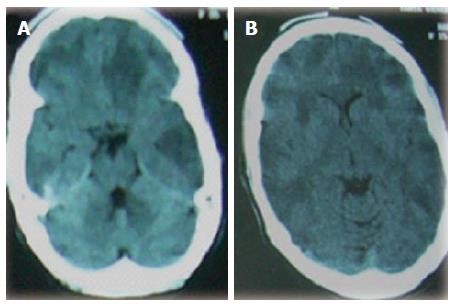
Figure 1 Cranial computed tomography brain showing axial views (A, B) with bilateral multifocal large hypodense lesions in the frontal, parietal, temporal and occipital lobes.
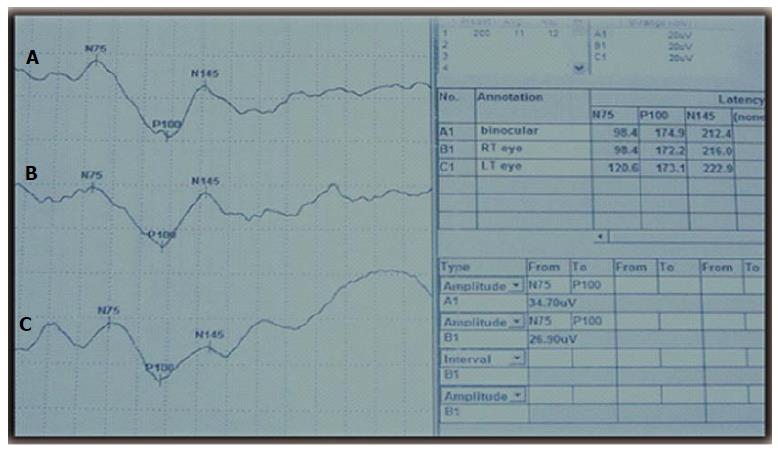
Figure 2 Visual evoked potentials showing prolonged absolute latencies of the P100 component of the right eye (A), left eye (B) and binocular (C).
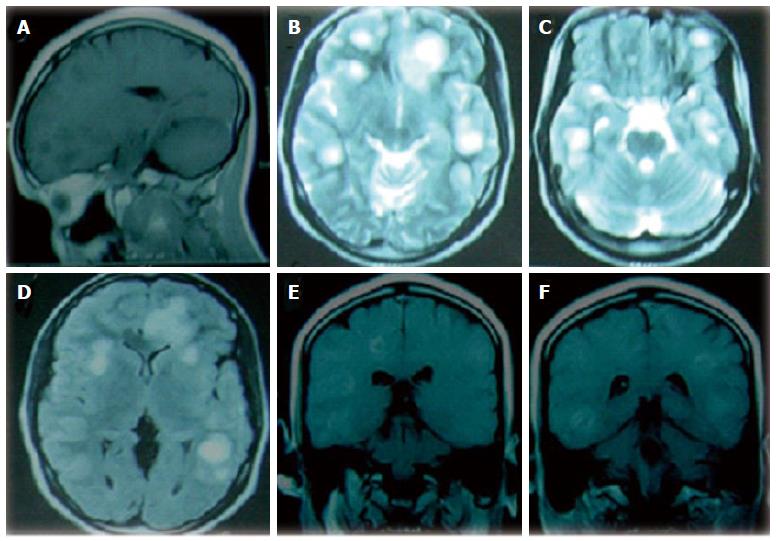
Figure 3 Cranial magnetic resonance imaging brain (on admission) showing (A) sagittal T1-weighted view with multiple hypointense lesions in the frontal and parietal regions, (B, C) axial T2-weighted images showing multifocal large hyperintense lesions in the frontal, parietal, temporal and occipital lobes, (D) axial fluid-attenuated inversion recovery-weighted image showing hyperintense lesions with minimal perifocal hypointense rim (edema), (E, F) coronal T1-weighted contrast enhanced views with patchy enhancement of the hypodense lesions in the temporal, parietal and frontal lobes bilaterally.
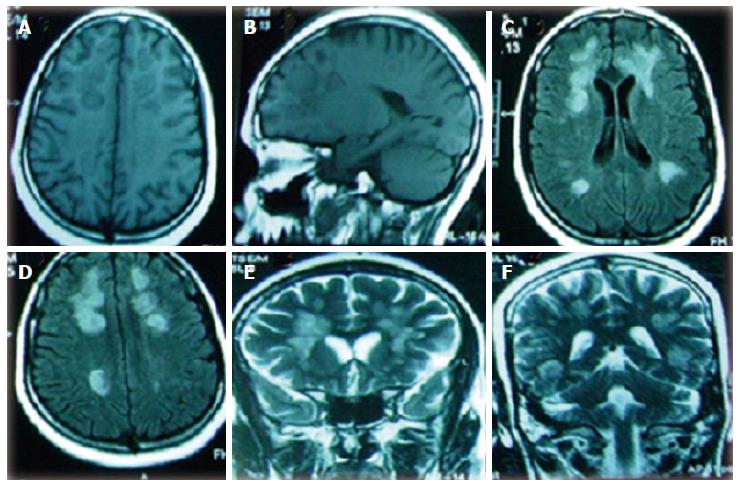
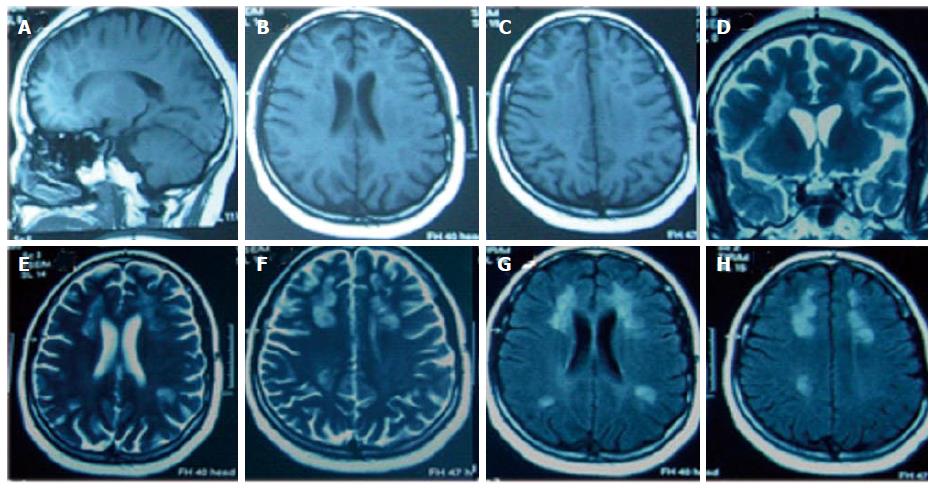
Figure 4 Cranial magnetic resonance imaging brain (after 6 mo of follow up) showing (A, B) axial and sagittal T1-weighted views with multiple hypointense lesions in the frontal, parietal, temporal and occipital regions, (C, D) axial fluid-attenuated inversion recovery-weighted images showing multifocal hyperintense lesions, (E, F) coronal T2-weighted images showing multifocal bilateral hyperintense lesions.
Figure 5 Cranial magnetic resonance imaging brain (after one year of follow up) showing (A, B, C) sagittal and axial T1-weighted views with bilateral multiple hypointense lesions in the frontal, parietal, temporal and occipital regions, (D, E, F) coronal and axial T2-weighted images showing multifocal bilateral hyperintense lesions, (G, H) axial fluid-attenuated inversion recovery-weighted images showing multifocal hyperintense lesions.
In all views, lesions had reduced sizes compared to those at 6 mo follow up.
The protocol of the study was approved by the local ethical committee of Assiut University Hospital which was in accordance of the principles of Helsinki and informed consent was obtained from the patient to publish her detailed clinical, laboratory and imaging data.
DISCUSSION
For our patient, tumefactive MS (or a tumor like demyelination) was established as diagnosis. Tumefactive MS is one of the atypical clinical and radiological presentations or variants described in adults with MS. It nearly represents 7% of cases with MS[8]. These variants are collectively named central nervous system inflammatory demyelinating diseases (CNS IDD) and include tumefactive MS[8-14], acute disseminated encephalomyelitis (ADEM)[15,16], Marburg’s MS[17], Balo Disease (or Balo concentric sclerosis)[18] and Devic’s disease or neuromyelitis optica[19]. In general, these variants have monophasic presentations and commonly aggressive or fulminant clinical course and do not show dissemination in time and space which is a typical feature for MS. Most commonly, these atypical aggressive attacks of variants of MS may present as the first demyelinating event in the course of MS. The management of these variants often prompts hospital admission and intensive care monitoring.
The diagnosis of tumefactive MS was done based on the acute onset (developed over days or weeks) of the condition, the predominance of cognitive impairment[8-14], the radiological evidence of multiple bilateral large rounded white matter lesions, absence of cortical involvement, absence of mass effect[9], patchy enhancement with gadolinium[14], regression with corticosteroids and lack of MRI evidence of new lesions on follow ups[8]. The severe cognitive impairment was due to great burden imposed by the large bilateral multifocal brain lesions. This also could explain the presence of aphasia and/or agnosia and/or apraxia (bilateral superficial and deep subcortical lesions). The marked diminution of vision in the patient was due to the presence of demyelinating optic neuropathy as evidenced by the VEPs, however, the possibility of the presence of field defects due to the involvement of the optic radiations in the temporal, parietal and occipital lobes of the cerebral hemispheres could not be excluded as additional cause of diminution of vision although visual field was not tested due to marked cognitive impairment. The enhanced lesions represented the areas of active inflammation, while the unenhanced lesions represented the chronic phase of the inflammatory process[20].
In general, tumefactive MS represents 1-2/1000 of cases of MS[21] with high frequency in adult females[14]. It is defined by the presence of single (frequent) or multiple large sized brain masses (≥ 2.0 cm in diameter)[9], associated with perilesional edema and mass effect[14]. The common clinical presentations of tumefactive MS include headache, cognitive abnormalities, mental confusion, impaired consciousness, aphasia, apraxia, cerebellar symptoms, visual field defects and/or seizures. With contrast, tumefactive demyelinating lesions usually appear as open-ring (directed toward the cortical surface or to the basal ganglia) or closed rings[14,20] or have diffuse, homogeneous, punctate, or concentric enhancement[14]. The CSF may have high levels of immunoglobulin G (IgG) index and oligoclonal bands. CSF may also be normal in fulminant conditions and short duration of the disease[22]. Abnormal visual (VEPs) and somatosensory-evoked potentials may present in 33%-60% of cases with tumefactive MS[14]. Acute treatments of tumefactive MS include intravenous methylprednisolone and/or plasma exchange, rituximab and natalizumab followed by immunomodulatory agents[12,23,24]. The prognosis and course of tumefactive MS remain controversial. Some studies reported clinical and radiological improvement of tumefactive demyelinating lesions with no future development of typical recurrent relapsing MS[8]. In contrast, few reported development of clinically definite MD[14,25,26].
The differential diagnosis related to the presented case may include: (1) other variants of MS as ADEM, Marburg’s MS and Balo concentric sclerosis; (2) CNS vasculitis; (3) intracranial infection/abscess or granuloma as tuberculoma; and (4) intracranial neoplastic SOLs as multifocal glioma, metastasis and primary CNS lymphoma (PCNSL).
ADEM is an acute multifocal monophasic inflammatory demyelinating disorder of the brain and spinal cord which should not progress beyond 3 mo. Although ADEM often occurs in the pediatric population, however adult cases have been reported. ADEM is often preceded by viral upper respiratory tract infection. ADEM is typically presented by encephalopathy. Other features may include vomiting, fever, headache, motor, sensory, and cerebellar symptoms, optic neuritis, seizures and aphasia[15,16]. Characteristic MRI features of ADEM include multifocal and diffuse hyperintense lesions in the gray and white matter of the brain and spinal cord in T2-weighted and FLAIR images and have no gadolinium enhancement[15,27]. Marburg’s MS is a very rare form of atypical MS which rapidly progress and often leads to rapid disability followed by death[17]. It is caused by the severe axonal loss. Balo concentric sclerosis is a rare and rapidly progressive variant of MS. It usually first appears in adulthood. Symptoms may progress rapidly over several weeks or more slowly over 2-3 years. Symptoms may include headache, seizures, gradual paralysis, involuntary muscle spasms , and cognitive loss. Balo concentric disease is so named because of the pattern of concentric layers formed by the damaged myelin tissues. Some patients have been known to spontaneously recover from this disease[18]. CNS vasculitis is inflammatory disease of the blood vessels of the brain or spinal cord. It is often secondary to connective tissue diseases or systemic autoimmune diseases as systemic lupus erythematosis (SLE), polyarteritis nodosa and rheumatoid arthritis and Behçet’s syndrome[28,29], but it can occur without associated systemic disease. Symptoms of CNS vasculitis may include headache, delirium, disorientation, forgetfulness or confusion, recurrent focal or generalized seizures, aphasia, apraxia, motor and sensory abnormalities, gait disorder, optic atrophy and encephalopathy. The MRI brain picture consistent with vasculitis include multiple ischemic brain lesions which appear as periventricular hyperintense dot-like areas in T2-weighted, FLAIR- and diffusion-weighted MRI imaging, multiple subcortical white matter hyperintense lesions in the frontal, temporal, parietal and occipital lobes, and abnormal enhancement of the leptomeninges. Magnetic resonance angiography may show string-of-beads stenosis of the carotid and vertebro-basilar arteries. Treatment of CNS vasculitis include immunotherapy as methylprednisolone, methotrexate, cyclophosphamide and intravenous immunoglobulins (IVIGs)[30]. Patients with intracranial infection/abscess/granulomas commonly have history of risk factors as immunocompromised state, dental abscess, pulmonary abscess, intravenous drug use, etc. Fever and abnormal labs (as high erythrocytic sedimentation rate or C-reactive protein) are clues for presence of infection. Presentation is usually of acute onset and secondary to symptoms of increased intracranial pressure (ICP), and seizures and focal neurological deficits are common. MRI-brain usually show ring enhancement which is often complete with regular margin[31]. Intracranial tuberculomas are relatively common in developing countries. It represents about 10%-30% of all intracranial masses of cases of infective intracranial SOLs. Seizures and focal neurological deficits are common clinical presentations. Systemic tuberculosis may be absent in up to 70% of cases. MRI-brain may show multiple intracranial enhancing SOLs particularly ring enhancement or target sign[32]. Intracranial neoplastic SOLs which mimic tumefactive MS include multifocal glioma, metastasis and PCNSL. Multifocal glioma usually presents insidiously with progressive neurologic deficit. Seizures are the common presentation in nearly one fourth of the patients of supratentorial lesions. Increased ICP is the common presentation of posterior fossa lesions[33]. Nearly fifteen percent of cases with cerebral metastasis have no previously known cancer. The primary cancers commonly involve the lung, breast, kidney, and gastrointestinal tract. Increased ICP, seizures and focal neurological deficits are common presenting manifestations[34]. PCNSL is a rare brain tumor that comprises only 1% of all intracranial neoplasms. Nearly 80% of cases of PCNSL have single supratentorial lesions. In one-third of the cases, PCNSL lesions are located deeply in the white matter while 10% are present in the posterior fossa. PCNSL is characterized by varied nonspecific neurologic deficits. The most common and early presenting manifestations are mental change, somnolence and paresis. Nearly 90% of cases with PCNSL have hyperdense or isodense lesions in CT brains and hypointense or isointense in T1-weighted MRI images. Only 40% have hyperintense lesions in T2-wighted images with mild edema and mass effect. Ring enhancement is more frequent in solitary lesions but meningeal enhancements is rare[35].
The atypical clinical and radiological presentations of MS may sometimes pose a considerable diagnostic difficulty. However and in spite of the aggressive course of its tumefactive variant, good prognosis may be seen in some patients.
COMMENTS
Case characteristics
A 25-year-old woman with history of acute dementia and blindness.
Clinical diagnosis
The patient had marked cognitive impairment, dysphasia, reduction in visual acuity and temporal pallor of the optic discs.
Differential diagnosis
Other variants of multiple sclerosis (MS) as ADEM, Marburg’s MS and Balo concentric sclerosis; central nervous system (CNS) vasculitis; intracranial infection/abscess/granuloma and neoplastic lesions as primary CNS lymphoma.
Imaging diagnosis
Magnetic resonance imaging-brain showed multifocal large (≥ 3 cm) white-matter hypointense lesions in T1W and hyperintense in T2W and fluid-attenuated inversion recovery images and patchy enhancement.
Pathological diagnosis
A demyelinating disease.
Treatment
Intravenous methylprednisolone in a dose of 1 g/d for 5 d was administered for two consecutive months followed by oral prednisolone in a dose of 80 mg twice daily was given for 1 mo. As no clinical improvement was observed, another dose of pulse steroid therapy was given which was also followed by 80 mg twice daily oral prednisolone for 9 mo followed by tapering of the steroid dose over the next year.
Related reports
Usually acute treatments of tumefactive MS include intravenous methylprednisolone and/or plasma exchange, galatiramer acetate, mitoxantrone and natalizumab followed by immunomodulatory agents as IFNβ-1a/b.
Term explanation
MS is a chronic immunologic disease in which inflammation, demyelination and axonal damage are the main pathologic features.
Experiences and lessons
In this case report, in spite of the fulminant presentation, good prognosis was seen with corticosteroids.
Peer-review
Interesting article on the scientific and practical level, text is well wrote and easily comprehensible with clear figures.