Published online Apr 16, 2015. doi: 10.12998/wjcc.v3.i4.371
Peer-review started: October 14, 2014
First decision: November 27, 2014
Revised: January 7, 2015
Accepted: January 18, 2015
Article in press: January 20, 2015
Published online: April 16, 2015
Processing time: 181 Days and 13.6 Hours
Dysbetalipoproteinemia is a rare familial dyslipidemia characterized by approximately equally elevated serum cholesterol and triglyceride levels due to accumulated remnant lipoproteins in apolipoprotein E2/E2 homozygotes. It is associated with an increased risk for premature cardiovascular disease. Thus, making a diagnosis of dysbetalipoproteinemia aids in assessing cardiovascular risk correctly and allows for genetic counseling. However, the diagnostic work-up can be challenging. Diagnosis of dysbetalipoproteinemia should be considered in patients mixed dyslipidemia when the apolipoprotein B concentration is relatively low in relation to the total cholesterol concentration or when there is significant disparity between the calculated low density lipoprotein (LDL) and directly measured LDL cholesterol concentrations. Other indices are also informative in the diagnostic process. We present herein two phenotypically different cases (a 44-year-old man with severe hypertriglyceridemia and a 49-year-old woman with mixed dyslipidemia) of genotypically proven familial dysbetalipoproteinemia and a diagnostic algorithm of the disease.
Core tip: Dysbetalipoproteinemia is associated with an increased risk for premature cardiovascular disease and its diagnosis may be challenging since its phenotype may significantly vary when specific environmental, hormonal and genetic factors that affect triglyceride (TG) metabolism co-exist. An algorithm with a number of dysbetalipoproteinemia indices may be helpful for the diagnosis of the disease and roughly equally elevated levels of both total cholesterol (TC) and TG and a low apolipoprotein B to TC ratio seem to comprise the two most helpful indices.
- Citation: Kei A, Miltiadous G, Bairaktari E, Hadjivassiliou M, Cariolou M, Elisaf M. Dysbetalipoproteinemia: Two cases report and a diagnostic algorithm. World J Clin Cases 2015; 3(4): 371-376
- URL: https://www.wjgnet.com/2307-8960/full/v3/i4/371.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i4.371
Apolipoprotein E (apoE) in plasma is mainly carried by chylomicrons, very-low-density lipoproteins (VLDL) and high-density lipoproteins (HDL). When associated with these lipoproteins, apoE serves as the ligand for the low density lipoprotein (LDL) receptor and the LDL-receptor related protein on the surface of hepatic cells[1]. In humans, three common apoE isoforms have been described, designated E2, E3, and E4[2,3]. ApoE2 and apoE4 differ from the more frequent apoE3 isoform by a single amino-acid substitution due to a single point mutation, conferring a more acidic or more basic charge to the protein. When compared with the apoE3 isoform, apoE2 has a markedly reduced affinity (< 1%) for the LDL receptor. Only a modest accumulation of cholesterol-enriched lipoprotein remnants of both hepatic and intestinal origin, or β-VLDL is observed in most apoE2/E2 homozygotes, which is not sufficient to cause an elevation of plasma cholesterol and triglyceride (TG) levels above normal. However, in individuals with predisposing genetic, hormonal, or environmental factors, this phenotype is associated with dysbetalipoproteinemia, also known as hyperlipoproteinemia type 3[2,4].
We present herein two phenotypically different cases of genotypically proven familial dysbetalipoproteinemia.
Blood samples for laboratory tests were obtained after a 12 h overnight fast. All serum laboratory measurements including fasting plasma glucose, creatinine, thyroid hormones, total cholesterol (TC), HDL cholesterol (HDL-C), and TG were determined enzymatically in the laboratory of the University Hospital of Ioannina using an Olympus AU 600 analyzer (Olympus Diagnostica GmbH, Hamburg, Germany). LDL cholesterol (LDL-C) was calculated using the Friedewald equation (provided that TGs were < 350 mg/dL (3.95 mmol/L). Apolipoproteins, serum and urinary proteins were measured with a Behring Nephelometer BN100 with reagents (antibodies and calibrators) from Dade Behring Holding GmbH (Liederbach, Germany). Antinuclear antibodies (MB Laboratories, Sidney, BC, Canada) were assessed by immunofluorescence. A commercial enzyme-linked immunosorbent assay was used to evaluate the levels of anti-extractable nuclear antigen (AESKU Diagnostics, Wendelsheim, Germany), while levels of serum globulins, C3 and C4 fractions of complement and rheumatoid factor were evaluated using nephelometry (Siemens Healthcare Diagnostics, Erlangen, Germany).
VLDL was isolated from plasma by two sequential ultracentrifugations according to the method of Gaw et al[5]. In brief, chylomicrons-deficient plasma firstly was prepared by plasma ultracentrifugation in a Beckman SW41 Ti rotor at 20000 rpm for 70 min at 23 °C. The chylomicrons fraction was carefully removed from the top of the tube and the chylomicrons-deficient fraction was next submitted to ultracentrifugation at density of d = 1.019 g/mL at 45000 rpm for 8 h at 14 °C. The VLDL fraction floating to the top of the tube was carefully collected.
DNA was extracted from the whole blood specimen according to standard procedures. ApoE genotyping was performed as described by Hixson and Vernier[6]. Polymerase chain reaction (PCR) was used to amplify a 244-bp sequence of the apoE gene, including the two polymorphic sites. The PCR product was then digested with the restriction enzyme Hha I and the different genotypes were detected after electrophoresis on 6% NuSieve agarose gel.
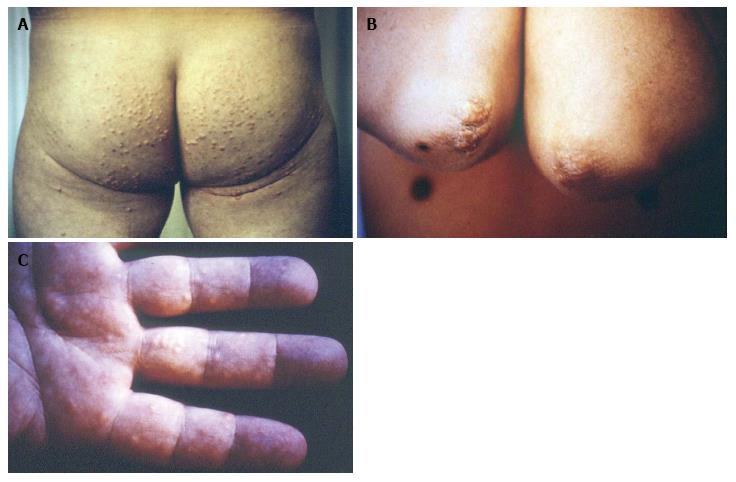
Two years earlier a 44-year-old Caucasian man had received the diagnosis of mixed dyslipidemia based on the following lipid profile; TC: 420 mg/dL, TG: 580 mg/dL, HDL-C: 36 mg/dL. He had no family history of dyslipidemia or established cardiovascular disease, while physical examination was unremarkable. Secondary causes of dyslipidemia were excluded by evaluation of thyroid and renal function, urinary protein excretion, serum protein electrophoresis, erythrocyte sedimentation rate and autoantibodies. A low-fat diet was recommended and patient was given gemfibrozil, 600 mg, twice a day. His lipid profile improved after 2 mo, but he stopped taking the drug and was lost to follow-up. At age 46, the patient had consulted a dermatologist because of the lesions shown at Figure 1 and was referred for serum lipid assessment. He consumed small amount of alcoholic beverages and had stopped smoking 5 years earlier. For the past 6 mo, he experienced symptoms that suggested intermittent claudication. A Doppler ultrasonic study revealed mild stenosis of left femoral artery.
Seen at Figure 1 are the characteristic for dysbetalipoproteinemia tuberous xanthomas over the patient’s elbows (they were also present on his knees) and the pathognomonic striated palmar xanthomas, while skin lesions typically associated with chylomicronemia, namely eruptive xanthomas, on his buttocks were also present (Figure 1). Laboratory assessment revealed: fasting plasma glucose: 300 mg/dL, TC: 1055 mg/dL, TG: 2900 mg/dL, HDL-C: 18 mg/dL, VLDL-C: 316 mg/dL, VLDL-TG: 831 mg/dL. The diagnosis of dysbetalipoproteinemia was verified by apoE2/E2 homozygosity genotype.
We suggested the patient to stop fat and alcohol consumption. He received metformin 850 mg twice a day and ciprofibrate 100 mg/d. Four weeks later the patient’s skin lesions had regressed significantly and serum laboratory parameters improved (fasting plasma glucose: 150 mg/dL, TC: 412 mg/dL, TG: 754 mg/dL, HDL-C: 27 mg/dL). The dosage of ciprofibrate was increased to 100 mg, twice a day and glimepiride 2 mg/d was added.
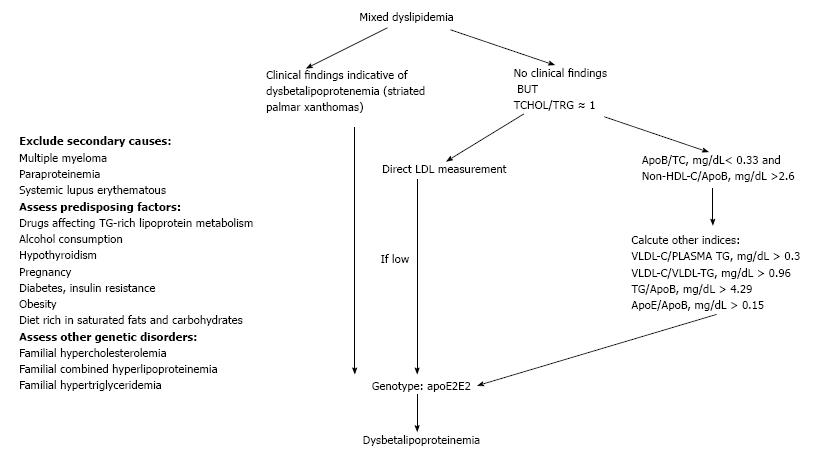
A 49-year-old Caucasian woman was referred to the Outpatient Lipid and Obesity Clinic of the University Hospital of Ioannina, Greece by her family doctor due to increased TC and TG levels. The patient denied any symptoms indicative of cardiovascular disease but her mother had been diagnosed with peripheral artery disease at the age of 40. In addition the patient had been diagnosed with breast cancer 2 years ago and her body mass index (BMI) was 28 kg/m2. She was on tamoxifen 20 mg/d and there had been no changes in her medication for the last 11 mo. Clinical examination revealed no pathologic findings, while her electrocardiogram was normal. Treatment naïve lipid profile analysis revealed elevated TC (325 mg/dL), TG (321 mg/dL), LDL-C (214 mg/dL), apoE (147 mg/dL) and lipoprotein a [Lp(a), 47.5 mg/dL] levels, while HDL-C (47 mg/dL), apoA1 (178 mg/dL) and apoB (77 mg/dL) levels were within normal range. Other secondary causes of hyperlipoproteinemia were excluded as in case 1 patient. Dysbetalipoproteinemia was suspected by the equally elevated levels of both TC and TG (TC/TG is approximately 1), before treatment initiation and the low apoB to TC ratio (< 0.33) (Figure 2).We first assessed a number of dysbetalipoproteinemia indices, while the apoE2/E2 homozygosity genotype verified the speculated diagnosis (Table 1, Figure 2).
| Index | Patient’s2 values | Indicative for dysbetali-poproteinemia values |
| TC/TG (mg/dL) | 1.01 | Approximately 1 |
| ApoB/TC (mg/dL) | 0.23 | < 0.33 |
| ApoB/non-HDL-C (mg/dL) | 0.27 | < 0.38 |
| VLDL-C/PLASMA TG (mg/dL) | 0.35 | > 0.3 |
| VLDL-C/VLDL-TG (mg/dL) | 1.06 | > 0.96 |
| TG/ApoB (mg/dL) | 4.40 | > 4.29 |
| ApoE/ApoB (mg/dL) | 0.20 | > 0.15 |
Patient was advised to switch to a diet low in saturated fats and carbohydrates. She received rosuvastatin 40 mg/d, ezetimibe 10 mg/d and fenofibrate 145 mg/d. Four weeks later patient’s lipid profile significantly improved (TC: 238 mg/dL, TG: 160 mg/dL, HDL-C: 50 mg/dL, LDL: 156 mg/dL).
Only untreated dysbetalipoproteinemia patients > 30-year-old, like case 1 patient, suffer from diagnostic skin lesions, including tuberous or tuberoeruptive xanthomas on the extensor surfaces of extremities (elbow, knees and buttocks)[7,8]. Striated palmar xanthomas are considered pathognomonic of dysbetalipoproteinemia, but they are not present in all patients (case 2 patient)[8].
Dysbetalipoproteinemia is characterized by increased serum TG and cholesterol rich lipoprotein remnants [mostly intermediate density lipoprotein (IDL) and chylomicron remnants], also known as β-VLDL particles[7,8]. As dysbetalipoproteinemia is associated with increased premature cardiovascular disease demanding aggressive therapeutic management, it is important for the physician to suspect the disease when a mixed dyslipidemia is further characterized by approximately equally elevated levels of both TC and TG (TC is approximately 250-450 mg/dL, TG is approximately 250-900 mg/dL) and a low apoB to TC ratio (< 0.33) (as it was the case with our second patient)[7,8]. The apoB to TC ratio represents the cholesterol in the circulating lipoproteins, but it does not include the cholesterol that circulates in HDL and other non-apoB-containing lipoproteins[9]. However, the apoB to non-HDL-C ratio was proven to be a less specific dysbetalipoproteinemia index compared with apoB to TC ratio[9]. When available, directly measured LDL-C is lower compared to that of calculated due to impaired conversion of VLDL to LDL[7,8,10]. In addition, an elevated VLDL cholesterol to total TG ratio (> 0.3) is indicative of dysbetalipoproteinemia and was also found in case 2 patient (Table 1)[7,8,10]. However, this ratio should not be used in normolipidemic subjects as they may have elevated ratios. Falsely low ratios, on the other hand, can be found in some dysbetalipoproteinemia patients who additionally have marked chylomicronemia and typical eruptive xanthomas (in the buttocks) as it was with case 1 patient. In this case, patient should be reassessed after several days on a low fat diet. In such cases another point distinguishing dysbetalipoproteinemia from type 5 hyperlipidemia is that the VLDL from a patient with dysbetalipoproteinemia is colored brown, whereas normal VLDL and that from type 2b and type 5 hyperlipidemia is white. Other dysbetalipoproteinemia indices have been also reported including elevated VLDL-C to VLDL-TG (> 0.96) as it represents high levels of cholesterol-enriched VLDL. In addition, elevated apoE to apoB has also been associated with dysbetalipoproteinemia and this index was elevated in our patient (Table 1)[9]. Last, the presence of a broad β band in electrophoresis is diagnostic but it is found in < 50% of cases[11].
Dysbetalipoproteinemia is observed in apoE2 homozygous persons when also a genetic or environmental risk factor for dyslipidemia is also present[7,8]. The disease generally presents after adulthood in men and menopause in women[7,8].
In most cases secondary factors are required for the expression of dysbetalipoproteinemia. These include additional genetic susceptibility variants, or other hormonal or environmental factors, such as obesity, type 2 diabetes, female gender, drugs affecting the metabolism of TG-rich lipoproteins, alcohol consumption or hypothyroidism (Table 2)[8,12]. Of note, case 1 patient was a diabetic man who consumed alcohol, while case 2 patient was an overweight woman who also received tamoxifen, which has been associated with disturbed TG metabolism. In fact tamoxifen, like estrogens, stimulates the synthesis and secretion from the liver of VLDL, which is the main circulating carrier of TGs, while it decreases VLDL and IDL catabolism as a result of decreasing lipoprotein and hepatic lipase activities. However, the drug can induce only modest elevations in serum TG levels in patients who have a normal lipoprotein metabolism, while it can cause marked hypertriglyceridemia in patients who have a defective TG-rich lipoproteins metabolism[13-15]. Moreover, some patients may possess additional genetic variants or mutations disturbing the metabolic role of apoE[7,8]. On the other hand, less than 5% of dysbetalipoproteinemia patients have dominant mutations in apoE, which per se induce mixed dyslipidemia[7,16]. These mutations impair both the chylomicron remnants and IDL particles uptake by liver and the conversion of VLDL and IDL to LDL particles[7]. Of note, patients with dysbetalipoproteinemia show a marked interindividual variation in the serum concentrations of cholesterol and TG, clinically presented as mixed dyslipidemia (case 2 patient) or chylomicromenia (case 1 patient).Furthermore a combination of the apoE2/2 phenotype and additional genetic factors associated with diseases like familial hypercholesterolemia, familial combined hyperlipoproteinemia, or familial hypertriglyceridemia, can determine the expression of dysbetalipoproteinemia[17]. According to the Dutch Lipid Clinic Network criteria proposed by the European Atherosclerosis Society, the diagnosis of familial hypercholesterolemia was not probable in both our patients[18].
| Environmental-hormonal | Genetic | Secondary dysbetalipoproteinemia |
| Drugs (corticosteroids, tamoxifen, retinoids, antipsychotics) | Familial hypercholesterolemia | Multiple myeloma |
| Alcohol consumption | Familial combined hypercholesterolemia | Paraproteinemia |
| Hypothyroidism | Reduced hepatic lipase activity | Systemic lupus erythematous |
| Pregnancy | Decreased lipoprotein lipase activity | |
| Diabetes, insulin resistance | ||
| Obesity | ||
| Diet rich in saturated fats and carbohydrates |
Noteworthy, it is important to exclude secondary causes of dysbetalipoproteinemia, including multiple myeloma, paraproteinemia and systemic lupus erythematous can mimic the disease, including the presence of typical xanthomas and the ultracentrifuge findings[19,20]. Thus, a detailed clinical and laboratory assessment is always required.
Dysbetalipoproteinemia patients have increased risk of both coronary artery disease and peripheral vascular disease, even though the LDL-C concentration is low[7,8]. Beta-VLDL is an atherogenic particle that rapidly transforms monocyte-macrophage cells to foam cells; the histologic hallmark of atherosclerosis and xanthomas[21]. Additionally, remnant lipoproteins induce endothelial plasminogen activator inhibitor I expression and activity in cultured aortic endothelial cells contributing to a prothrombotic state[22]. Males with homozygosity for the ApoE2 isoform present with coronary disease at their 4th or 5th decade of their life and there is a predisposition for peripheral vascular disease in these patients[23]. Last, lipoprotein glomerulopathy and pancreatitis in severe hypertriglyceridemic patients with dysbetalipoproteinemia have also been described[24].
Treatment of dysbetalipoproteinemia is the same as for hypertriglyceridemia. Weight loss, diet fat restriction and treatment of secondary factors, such as diabetes and hypothyroidism are important for all dysbetalipoproteinemia patients[7]. In addition, administration of fibrates, statins, omega-3 fatty acids and niacin or their combinations is very effective. However, it has to be underlined that fibrates, with or without statin, seem to comprise the cornerstone of dysbetalipoproteinemia treatment[25].
In conclusion, physician should keep in mind that dysbetalipoproteinemia may present as chylomicronemia when other genetic or environmental causes affecting TG metabolism co-exist. Moreover, dysbetalipoproteinemia has to be suspected in all mixed dyslipidemia cases with equally elevated TC and TG levels (TC/TG = 1) (Figure 2).
The two patients presented with dissimilar lipid profile; one presented with extremely high triglycerides (TG) levels and the other presented with equally elevated levels of total cholesterol (TC) and TG.
The physical signs of the two cases were also dissimilar; one patient presented with tuberous and eruptive xanthomas, while the other patient had no skin lesions.
Type 5 dyslipidemia, chylomicronemia, secondary causes of mixed dyslipidemia and dysbetalipoproteinemia.
The first patient had the following lipid profile: TC: 1055 mg/dL, TG: 2900 mg/dL, high density lipoprotein cholesterol: 18 mg/dL, while the second patient had the following lipid profile; TC: 325 mg/dL, TG: 321 mg/dL, low density lipoprotein cholesterol (LDL-C): 214 mg/dL.
The diagnosis of dysbetalipoproteinemia was verified by Apolipoprotein E2 (apoE2)/E2 homozygosity genotype in both patients.
Fibrate with or without statin improved lipid profile in both patients.
Dysbetalipoproteinemia is seen in approximately 1 in 10000 people.
Dysbetalipoproteinemia is a rare familial disease characterized by marked elevations of serum cholesterol and triglyceride levels caused by an accumulation of remnant lipoproteins in apolipoprotein E2/E2 homozygotes.
This case report presents the clinical characteristics and lipid profile of dysbetalipoproteinemia and also suggests a diagnostic algorithm. The authors recommend that diagnosis of dysbetalipoproteinemia should be considered in patients mixed dyslipidemia when the apolipoprotein B concentration is relatively low in relation to the total cholesterol concentration or when there is significant disparity between the calculated low LDL-C and directly measured LDL-C concentrations.
This is a nice article that deserves be published.
P- Reviewer: Paraskevas KI S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Mahley RW, Innerarity TL. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983;737:197-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 583] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 2. | Utermann G. Apolipoprotein E mutants, hyperlipidemia and arteriosclerosis. Adv Exp Med Biol. 1985;183:173-188. [PubMed] |
| 3. | Zannis VI, Just PW, Breslow JL. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981;33:11-24. [PubMed] |
| 4. | Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1257] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 5. | Gaw APC, Shepherd J. Lipoprotein Turnover and Metabolism In Lipoprotein Analysis. A Practical Approach. New York: Oxford University Press 1992; 128-133. |
| 6. | Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545-548. [PubMed] |
| 7. | Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 384] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Mahley RW, Huang Y, Rall SC. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res. 1999;40:1933-1949. [PubMed] |
| 9. | Blom DJ, O’Neill FH, Marais AD. Screening for dysbetalipoproteinemia by plasma cholesterol and apolipoprotein B concentrations. Clin Chem. 2005;51:904-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 11. | Blom DJ, Byrnes P, Jones S, Marais AD. Non-denaturing polyacrylamide gradient gel electrophoresis for the diagnosis of dysbetalipoproteinemia. J Lipid Res. 2003;44:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Schaefer EJ, Lamon-Fava S, Johnson S, Ordovas JM, Schaefer MM, Castelli WP, Wilson PW. Effects of gender and menopausal status on the association of apolipoprotein E phenotype with plasma lipoprotein levels. Results from the Framingham Offspring Study. Arterioscler Thromb. 1994;14:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Elisaf M, Bairaktari E, Pavlidis N. The influence of tamoxifen on serum triglycerides. Breast. 2000;9:238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Filippatos TD, Liberopoulos EN, Pavlidis N, Elisaf MS, Mikhailidis DP. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev. 2009;35:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Milionis HJ, Liberopoulos EN, Elisaf MS. Tamoxifen-induced hypertriglyceridemia in association with diabetes mellitus. Diabetes Metab. 2001;27:160-163. [PubMed] |
| 16. | Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 17. | Utermann G, Vogelberg KH, Steinmetz A, Schoenborn W, Pruin N, Jaeschke M, Hees M, Canzler H. Polymorphism of apolipoprotein E. II. Genetics of hyperlipoproteinemia type III. Clin Genet. 1979;15:37-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 150] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478-390a. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1632] [Cited by in RCA: 1972] [Article Influence: 164.3] [Reference Citation Analysis (0)] |
| 19. | Chee L, Spearing RL, Morris CM, McDonald M, Hanrahan V, Ebbett A, Scott R, Florkowski C, Walmsley T, Patton WN. Acquired myeloma-associated Type III hyperlipidaemia treated by nonmyeloablative HLA-identical sibling allogeneic stem cell transplant using a donor with essential thrombocythaemia (ET): evidence of engraftment without manifestation of ET in recipient. Bone Marrow Transplant. 2005;35:1213-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Burnside NJ, Alberta L, Robinson-Bostom L, Bostom A. Type III hyperlipoproteinemia with xanthomas and multiple myeloma. J Am Acad Dermatol. 2005;53:S281-S284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Whitman SC, Hazen SL, Miller DB, Hegele RA, Heinecke JW, Huff MW. Modification of type III VLDL, their remnants, and VLDL from ApoE-knockout mice by p-hydroxyphenylacetaldehyde, a product of myeloperoxidase activity, causes marked cholesteryl ester accumulation in macrophages. Arterioscler Thromb Vasc Biol. 1999;19:1238-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Sawka AM, Singh RJ, Hiddinga HJ, McConnell JP, Eberhardt NL, Caplice NM, O’Brien T. Remnant lipoproteins induce endothelial plasminogen activator inhibitor-1. Biochem Biophys Res Commun. 2001;285:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Hopkins PN, Wu LL, Hunt SC, Brinton EA. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J Am Coll Cardiol. 2005;45:1003-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Saito T, Oikawa S, Sato H, Sato T, Ito S, Sasaki J. Lipoprotein glomerulopathy: significance of lipoprotein and ultrastructural features. Kidney Int Suppl. 1999;71:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Marais AD, Solomon GA, Blom DJ. Dysbetalipoproteinaemia: a mixed hyperlipidaemia of remnant lipoproteins due to mutations in apolipoprotein E. Crit Rev Clin Lab Sci. 2014;51:46-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |