Published online Mar 16, 2015. doi: 10.12998/wjcc.v3.i3.318
Peer-review started: July 8, 2014
First decision: September 30, 2014
Revised: December 11, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: March 16, 2015
Processing time: 249 Days and 4.2 Hours
May-Thurner syndrome (MTS) also termed iliocaval compression or Cockett-Thomas syndrome is a common, although rarely diagnosed, condition in which the patient has an anatomical variant wherein the right common iliac artery overlies and compresses the left common iliac vein against the fifth lumbar spine resulting in increased risk of iliofemoral deep venous thrombosis. This variant has been shown to be present in over 23% of the population but most go undetected. We present a patient with MTS who developed high output cardiac failure due to an iatrogenic iliac fistula. The patient underwent an extensive workup for a left to right shunt including MRI and arterial duplex in the vascular lab. He was ultimately found to have a 2.1 cm left common iliac artery aneurysm and history of common iliac stent. We took the patient to the operating room for aortogram with placement of an endovascular plug of the left internal iliac artery and aorto-bi-iliac stent graft placement with CO2 and IV contrast. Subsequently the patient underwent successful stent placement in the area that was compressed followed by 6 mo of anticoagulation with warfarin. The flow from the fistula decreased significantly.
Core tip: To our knowledge, we describe the first case of high output cardiac failure due to iatrogenic iliac fistula and its management in the setting of May-Thurner syndrome (MTS). In our case, an iatrogenic iliac fistula resulted because of prior stent placement in left iliac vein to prevent deep venous thrombosis (DVT) secondary to MTS. We favored aorto-bi-iliac stent graft placement to prevent the fistula from leaking. In our case, the prior vascular stent placement was a clue to search for the fistula. It is important to note that stent placement to prevent DVT in MTS may result in iatrogenic fistula formation.
- Citation: Singh S, Singh S, Jyothimallika J, Lynch TJ. May-Thurner syndrome: High output cardiac failure as a result of iatrogenic iliac fistula. World J Clin Cases 2015; 3(3): 318-321
- URL: https://www.wjgnet.com/2307-8960/full/v3/i3/318.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i3.318
May Thurner syndrome (MTS) or iliocaval compression or Cockett-Thomas syndrome is a fairly common well recognized and rarely diagnosed condition. McMurrich first described this anatomical variant in 1908 and believed the variant was the result of “congenital adhesions” in the common iliac veins. May and Thurner published this syndrome in 1957 and it was widely recognized as MTS in United States. In Europe, however, Cockett, a British vascular surgeon and Thomas published the condition in 1965 and it was termed Cockett-Thomas Syndrome. May and Thurner postulated that the chronic pulsations of the overlying right iliac artery led to development of a “spur” in the vein wall and that this spur would result in partial venous obstruction.
A 61 years old male was admitted to our cardiology service for shortness of breath associated with hemodynamic instability (systolic blood pressure, 95mm Hg and diastolic blood pressure, 59 mmHg). His past medical history is significant for hypertension, congestive heart failure, coronary artery disease (post coronary artery bypass graft - 1999), MTS [treated with left iliac vein stent in absence of PE and deep venous thrombosis (DVT) in 2001], chronic pulmonary embolism, protein C deficiency, restrictive lung disease and mild obstructive lung disease. Additionally, the patient smokes half a pack daily for 30 years.
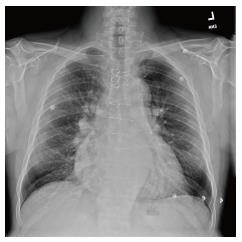
His immediate physical examination revealed hypotension (105/46 mmHg), jugular venous distension of 6 cm, 2/6 pansystolic murmur at the apex, and bilateral pedal edema was noted. Abdomen was soft and non-tender but was distended with normoactive bowel sounds and and liver edge 1 cm below the costal margin. A chest x ray showed mild cardiomegaly with prominence of the central pulmonary arteries (Figure 1). Initial BNP and fibrinogen levels were both elevated (166.7 hh pg/mL and 553 mg/dL). Other blood tests supported renal insufficiency (creatinine 1.68 mg/dl). Echocardiogram revealed diffuse hypokinesia with a low ejection fraction (35%), cardiac output (11.5 L/min), cardiac index (5.54 L/min per square), end diastolic volume (183 mL), end Systolic volume (101 mL), concentric left ventricular hypertrophy, abnormal diastolic relaxation and mild to moderate mitral regurgitation.
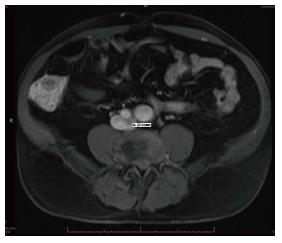
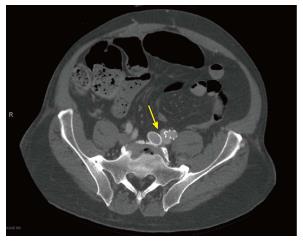
Vascular surgery was consulted for evaluation of a possible pelvic shunt/fistula. After extensive workup for a left to right shunt including MRI and arterial duplex, the patient was found to have a 2.1 cm left common iliac artery aneurysm (21.4 mm) (Figure 2). An Inferior Vena Cava duplex and computed tomography (CT) of the pelvis found evidence of atriovenous fistula at the left iliac vein (discovered 11 years after the initial stent was placed in 2001) (Figure 3).
The patient was taken for an aortogram and heparinized with 5000 units of IV heparin. The right common femoral artery was then accessed and a 5-French sheath was advanced over the wire. An Omniflush catheter was then positioned at the level of L1. The patient had evidence of a high volume fistula from within the left common and hypogastric artery on CO2 aortogram. Given this finding, an additional magnified view was obtained with the Omniflush catheter positioned at the aortic bifurcation. The Glidewire and Omniflush catheter were then used to select the left hypogastric artery. A 6-French Balkan sheath was brought up and over Magic Torque wire and into the left hypogastric artery. The Amplatz 16 mm plug was then advanced into the distal hypogastric artery. This appeared to be distal to any evidence of the fistula. The plug was then deployed in proper position. Next, a marker pigtail catheter was advanced from the right groin. The left groin cutdown was then performed, using a transverse incision. A 5-French sheath was advanced over the wire. A J-wire was advanced into the descending thoracic aorta. This was exchanged for a Lunderquist wire over the stiff wire. A CO2 aortogram was again performed. This demonstrated the level of the renal arteries. The main body device, Cook Zenith TFB-28-74 was brought over the Lunderquist wire in the left groin. This was deployed down to the level of the contralateral gate. The pigtail catheter was then pulled down and the top cap was deployed securing the position of the graft. Next, a Kumpe catheter was used to select the contralateral limb from the right femoral sheath. The pigtail catheter was spun confirming proper position. An Amplatz wire was advanced from the right groin. A retrograde injection of contrast was performed from the right groin. Next, the contralateral limb, which was a Cook ZSLE-20-39-ZT, was then deployed with care taken to preserve flow to the right hypogastric artery. Next, the remainder of the main body device was deployed from the left groin. The top cap was then retrieved, and the sheath was removed. Due to the incompetence of a valve, the sheath was then replaced with a Gore DrySeal 18-French Sheath. Next, a retrograde injection of contrast was performed from the left groin. The ipsilateral limb, which was a Cook ZSLE-13-90-ZT was brought onto the field and prepped. This was deployed with care taken to ensure 5 cm of overlap into the left external iliac artery. A 32-French Coda balloon was then used to balloon the proximal and distal sites of fixation, as well as all zones of overlap. Wires were exchanged for a soft wire. This demonstrated evidence of a persistent flow within the fistula that appeared to be less than previous. A completion aortogram was performed. By the end of the procedure cardiac output and cardiac index returned to normal and patient remained stable. Flow through the fistula stopped.
MTS is a fairly common, well recognized and rarely diagnosed condition[1]. McMurrich believed the variant was the result of “congenital adhesions” in the common iliac veins[2]. May and Thurner published this syndrome in 1957 and it was widely recognized as MTS in United States whereas Cockett and Thomas published it in 1965 and called the same condition Cockett-Thomas Syndrome in Europe[3]. May and Thurner postulated that the chronic pulsations of the overlying right iliac artery led to development of a “spur” in the vein wall and that this spur would result in partial venous obstruction[4]. Of 430 cadavers, 22% were diagnosed with spurs on left side which is eight times more common than on the right. This came a century after Virchow (1851) first described that thrombosis on the left side was five times more common than on the right side[5]. More recently Kibbe et al[6] demonstrated via CT the incidence of MTS in asymptomatic patients that correlated with autopsy results reported in the ealy half of twentieth century.
The goal of treatment of MTS is to reduce symptoms and to reduce the risk of complications. The majority of treatments are geared towards treating DVT. The first known report of treatment of MTS solely by endovascular means was by Berger et al[7] in 1995, who successfully placed a venous stent to relieve iliac compression. The initial step in the treatment of DVT in the setting of MTS is thrombectomy with stent placement[8]. Vena Cava filters may be a treatment option for select patients who cannot take anticoagulant medications. Vena Cava filters may not always be used in the treatment of MTS but are used to prevent complications of DVT.
To our knowledge, ours is the first case to report high output cardiac failure due to iatrogenic iliac fistula and its management in MTS. In this case, an iatrogenic iliac fistula resulted because of prior stent placement in left iliac vein to prevent DVT secondary to MTS. We favored aorto-bi-iliac stent graft placement to prevent the fistula from leaking. We managed to successfully reduce cardiac output (11.5 L/min to 8.3 L/min) and cardiac index (5.54 L/min per m sq to 4.0 L/min per squre).
It is important for the practicing physician to note that the identification of high output cardiac failure should lead to a search for the source. In our case, the prior vascular stent placement was a clue to search for the fistula. It is also important to note that stent placement to prevent DVT in MTS can result in iatrogenic fistula.
We thank Prashant Bhensdadia (MD) (Division of Cardiology, Wake Forest Health, 27157), for bringing to notice this interesting case.
The patient presented with shortness of breath and bilateral pedal edema.
The patient was found to high output cardiac failure due to iliac fistula resulting from prior management of May-Thurner syndrome.
Based on patient history, physical exam, and imaging the authors were able to narrow down on the diagnosis by ruling out severe anemia, paget’s disease of bone, hyperthyroidism and beriberi.
The images were obtained by angiography and chest X ray.
The patient underwent angiography and stent placement.
Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg 1995; 21: 510-514.
It is also important to note that stent placement to prevent deep venous thrombosis in May-Thurner syndrome can result in iatrogenic fistula.
This is an interesting case. English well written, understandable and easily readable.
P- Reviewer: Setacci C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Peters M, Syed RK, Katz M, Moscona J, Press C, Nijjar V, Bisharat M, Baldwin D. May-Thurner syndrome: a not so uncommon cause of a common condition. Proc (Bayl Univ Med Cent). 2012;25:231-233. [PubMed] |
| 2. | McMurrich JP. The occurrence of congenital adhesions in the common iliac veins and their relation to thrombosis of the femoral and iliac veins. Am J Med Sci. 1908;135:342-346. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8:419-427. [PubMed] |
| 5. | Virchow R; Uber die Erweiterung kleiner Gefasse. Arch Path Anat. 1851;3:427-462. |
| 6. | Kibbe MR, Ujiki M, Goodwina AL, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. 2003;. |
| 7. | Berger A, Jaffe JW, York TN. Iliac compression syndrome treated with stent placement. J Vasc Surg. 1995;21:510-514. [PubMed] |
| 8. | Mickley V, Schwagierek R, Rilinger N, Görich J, Sunder-Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998;28:492-497. [PubMed] |