Published online Mar 16, 2015. doi: 10.12998/wjcc.v3.i3.206
Peer-review started: October 24, 2014
First decision: December 12, 2014
Revised: December 28, 2014
Accepted: January 15, 2015
Article in press: January 19, 2015
Published online: March 16, 2015
Processing time: 141 Days and 14.4 Hours
Traditional right ventricular (RV) apical pacing has been associated with heart failure, atrial fibrillation and increased mortality. To avoid the negative consequences of RV apical pacing different strategies have been developed, among these a series of pacing algorithms designed to minimize RV pacing. These functions are particularly useful when there is not the need for continuous RV pacing: intermittent atrio-ventricular blocks and, mainly, sinus node disease. However, in order to avoid RV pacing, the operational features of these algorithms may lead to adverse (often under-appreciated) consequences in some patients. We describe a case of a patient with sinus node disease, in whom right atrial only pacing involved long atrio-ventricular delay to allow intrinsic ventricular conduction, which led to symptomatic hypotension that could be overcome only by “forcing” also right ventricular apical pacing. We subsequently discuss this case in the context of current available literature.
Core tip: Right ventricular apical pacing has been associated with worse outcome so a series of pacing algorithms have been designed to minimize it. However the operational features of these algorithms may lead to adverse consequences in some patients. We describe a case of a patient with sinus node disease, in whom right atrial only pacing involved long atrio-ventricular delay to allow intrinsic ventricular conduction, which led to symptomatic hypotension that could be overcome only by “forcing” right ventricular apical pacing. We subsequently discuss this case in the context of current available literature.
- Citation: Maria ED, Olaru A, Cappelli S. Minimizing right ventricular pacing in sinus node disease: Sometimes the cure is worse than the disease. World J Clin Cases 2015; 3(3): 206-209
- URL: https://www.wjgnet.com/2307-8960/full/v3/i3/206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i3.206
Traditional right ventricular (RV) apical pacing has been associated with heart failure, atrial fibrillation and increased mortality[1]. Dyssynchronous electrical activation of the heart from pacing creates deleterious myocardial fiber strain, mechanically inefficient contraction and adverse left ventricular (LV) remodeling with a progressive, dose-related, decline in pump function that is more evident in patients with an already compromised cardiac function at baseline[2]. To avoid the negative consequences of RV apical pacing different strategies have been developed. Pacing from alternative RV sites (septum, outflow tract, His bundle) is a promising option but, up to now, studies comparing apical to non-apical pacing with regard to hemodynamic, echocardiographic and long-term LV systolic function have been conflicting, failing to demonstrate a clear benefit[3,4]. Biventricular pacing can be considered in selected cases, i.e., patients with atrio-ventricular (AV) block and LV ejection fraction < 35%, but it is not a first choice in the majority of patients candidates to receive a pacemaker. Contemporary pacemakers, from different manufacturers, include sophisticated algorithms designed to minimize ventricular pacing with the aim to reduce the incidence of atrial fibrillation and heart failure[5]. These functions are particularly useful when there is not the need for continuous RV pacing, that is in patients with intermittent AV blocks and, mainly, sinus node disease (SND). However, in order to avoid RV pacing, the operational features of these algorithms involve long AV delay to allow intrinsic conduction, which may lead to adverse (often under-appreciated) consequences in some patients[5,6].
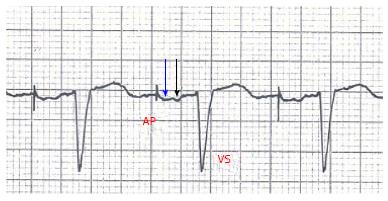
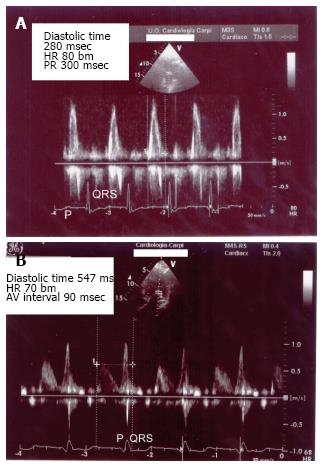
We describe the case of a 85-year-old man implanted with a dual chamber pacemaker (Adapta DR Medtronic, leads positioned in right atrial appendage and RV apex) because of sinus node disease/bradi-tachi syndrome. He did not take any drug. Baseline ECG showed sinus bradycardia (35-40 beats per minute), normal P wave morphology, PR interval 180 msec, QRS 115 msec. Echocardiogram revealed moderate LV hypertrophy, ejection fraction 50%; diastolic pattern (pulsed wave mitral Doppler) showed abnormal relaxation with an adequate filling time in sinus rhythm. Before pacemaker implant he was symptomatic for palpitations, easy fatiguability, dizziness, vertigo, but no syncopal episode was described. Pacemaker was programmed DDDR 60-120 bm, Managed Ventricular Pacing (MVP)™ turned ON to avoid unnecessary RV pacing; ECG after implant showed atrial-based pacing with spontaneous ventricular activation (AP-VS) (Figure 1). Few days after hospital discharge the patient returned to our attention for episodes of near-syncope and falls occurring shortly after the passage from supine to upright position. We documented a symptomatic orthostatic hypotension with a sudden drop in systolic and diastolic blood pressure (SBP drop 30-40 mmHg and DBP drop 15-20 mmHg); during the episodes heart rate increased to about 80-90 beats per minute due to sensor-driven atrial pacing (with spontaneous ventricular activation). There was no obvious cause to justify these episodes, in particular different etiologies of orthostatic hypotension (neurogenic, non-neurogenic, drug/toxins effect) had been excluded; pacemaker did not show any malfunction. So we repeated an echocardiogram, recording diastolic filling during the episodes of symptomatic orthostatic hypotension: we found E/A wave fusion with a particularly short diastolic filling time (about 280 msec at 80 beats per minute) (Figure 2A). We also noticed that at ECG, during atrial-based pacing, AP-VS interval was about 280-300 msec and right atrial stimulus artifact was followed by a first deflection corresponding to right atrial depolarization (white arrow in Figure 1) and then a second deflection corresponding to left atrial depolarization (black arrow in Figure 1). We also tried to program the pacemaker in AAI mode with a fixed rate but the results were the same compared to MVP™. All these features suggested us that atrial-based pacing was responsible of an abnormal prolongation of AV interval (with E/A fusion), likely associated with intraatrial and interatrial conduction delay, with symptoms (near-syncope) and orthostatic exacerbation similar to “pacemaker syndrome”. However, by definition pacemaker syndrome occurs when there is atrial systole during ventricular systole while E/A fusion seen in our case is a diastolic filling issue. So MVP™ was turned OFF and we optimized the programmed AV interval to 90 msec (“forcing” RV pacing) in order to ensure an adequate echocardiographic diastolic filling time, with a good separation of E and A waves at pulsed wave mitral Doppler (diastolic filling time 547 msec at 70 beats per minute) (Figure 2B). Since the first day after reprogramming the device, the episodes of orthostatic hypotension did not occur anymore; we tested the patient with an “orthostatic stress test” (a sudden change from supine to upright position while monitoring blood pressure, heart rate and diastolic filling pattern) and neither hypotension nor symptoms occurred during AP-VP paced rhythm.
Diastole begins soon after the end of systolic ejection (aortic valve closure) and includes LV pressure fall, rapid filling, diastasis and atrial contraction. Diastolic filling and cardiac output are strictly linked and the optimal performance of the LV depends on the alternation between a compliant chamber in diastole (LV filling from a low atrial pressure) and a stiff chamber in systole (ejection of the stroke volume at arterial pressures). When passing from supine to upright position there is a venous pooling in the lower extremities and splanchnic circulation as a result of the gravitational change. The consequent decrease of venous return to the heart leads to a transient reduction of ventricular filling, cardiac output and blood pressure. As compensatory mechanisms sympathetic tone increases and parasympathetic activity decreases: venous return, heart rate and vascular resistance they all increase with the aim of maintaining cardiac output and blood pressure. When one or more of these compensatory mechanisms fail orthostatic hypotension can occur.
Several factors contribute to the clinical, ECG and echo findings in our patient. First of all pacing from right atrial appendage led to a delay in interatrial and intraatrial conduction as manifested by a “wide” P wave following stimulus artifact, with two distinct deflections corresponding to right and left atrial depolarization (Figure 1), while P wave morphology in spontaneous sinus rhythm was completely normal. As a consequence, a long AV delay occurred (AP-VS 300 msec) that was likely and mainly the consequence of inter/intra atrial conduction delay (rather than a true nodal/hisian delay, PR interval being normal during non-paced rhythm). This kind of “pseudo first degree AV block” pushed the A wave toward E wave: E/A fusion occurred so diastolic filling time was abnormally short. During orthostatic challenge the inability to fill the ventricle, because of this diastolic impairment, finally led to symptomatic hypotension with near-syncope. The only way to restore an adequate filling time was to optimize AV delay, but this involved to “force” RV pacing.
MVP™ provides an atrial-based pacing (AAI/R) with ventricular backup at an AV delay of 80 msec in absence of a ventricular sensed event following an atrial sensed or paced event. When loss of AV conduction persists (two out four non-refractory AA intervals without ventricular sensed events) the pacemaker switches to DDD/R mode at the programmed lower rate and AV delay. The algorithm, then, performs regular checks of AV conduction and switches back to AAI/R mode if possible[5]. The MVP™ tolerates markedly prolonged AV delay which can adversely affect cardiovascular hemodynamics, reducing atrial contribution to ventricular filling and favoring diastolic mitral regurgitation[5,6]. In general algorithms designed to minimize ventricular pacing operate by prolonging the AV interval with hysteresis or by switching between DDD and AAI modes; the operative features differ between manufacturers but all of them carry the risk of AV decoupling (defined as > 40% of AV intervals over 300 msec) even when baseline PR interval is normal. To prevent this adverse effect some manufactures have incorporated in their algorithms a maximum tolerated AV delay (350 msec in Ventricular Intrinsic Preference™ by St Jude Medical; 350 msec atrial sensed and 450 msec atrial paced in AAISafeR2™ by Sorin Group): if AV delay exceeds these limits, the device switches to DDD mode.
Atrial pacing “per se” increases AV delay: pacing from right atrial appendage can provoke marked alterations in interatrial and intraatrial impulse propagation that impairs coordinated activation and can also favor atrial fibrillation[7]. In the DANPACE trial[8], that compared AAI and DDD pacing in SND, atrial-based pacing significantly increased the risk of paroxysmal atrial fibrillation [28.4% in AAI group vs 23% in DDD group; hazard ratio (HR) 1.27; P = 0.024]. In a study AAI-R based pacing, in patients with SND and normal baseline PR interval, induced a clinically significant lengthening of AV conduction time, with a paradoxical increase of AV conduction during exercise in 66% of cases (that was predicted by use of antiarrhythmic class Ic/III drugs)[9]. Moreover in 23%-58% of SND patients AV conduction is already impaired at baseline, two thirds of these patients having a first degree AV block; the optimal pacing mode in these subgroup is not determined. In a comparison study between conventional dual chamber pacing and minimal ventricular pacing mode there was no significant difference in terms of functional capacity assessed by cardiopulmonary test, quality of life and echocardiographic parameters of systolic/diastolic function; it was concluded that sequential AV pacing may be a reasonable choice for patients with SND and prolonged PR interval[10].
Alternative atrial pacing sites have also been studied: high and low interatrial septum, Bachmann bundle, lateral free wall and combinations of these sites; the concept was to improve atrial hemodynamics by reducing total atrial activation time. Although several small studies indicated that some alternative sites could help to prevent atrial fibrillation, randomized trials did not show benefit in the long term[11].
The attempt to minimize RV pacing, at expense of AV synchrony, can be particularly deleterious in patient with heart failure. In the INTRINSIC RV trial[12] patients indicated for ICD implant were randomized to dual chamber pacing with AV Search Hysteresis™ or single chamber VVI pacing 40 bpm. Patients with 10%-19% RV pacing had the most favorable outcome, while the risk of clinical events (mainly heart failure decompensation) in the 0%-9% RV pacing group was as high as the 40%-49% RV pacing group. So some ventricular pacing may be necessary, even if the optimal balance between AV synchrony and intraventricular dyssynchrony induced by RV pacing varies between patients and is not simple to define.
This statement is to certify that all authors have seen and approved the manuscript being submitted, have contributed significantly to the work, attest to the validity and legitimacy of the data and its interpretation, and agree to its submission to the Journal. We attest that the article is the Authors’ original work, has not received prior publication and is not under consideration for publication elsewhere. On behalf of all co-authors, the corresponding Author shall bear full responsibility for the submission. Any changes to the list of authors, including changes in order, additions or removals will require the submission of a new author agreement form approved and signed by all the original and added submitting authors. Patient’s consent was obtained. The authors report no relationships that could be construed as a conflict of interest.
P- Reviewer: Anan R, Trohman R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932-2937. [PubMed] |
| 2. | Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol. 1990;259:H300-H308. [PubMed] |
| 3. | Cano O, Osca J, Sancho-Tello MJ, Sánchez JM, Ortiz V, Castro JE, Salvador A, Olagüe J. Comparison of effectiveness of right ventricular septal pacing versus right ventricular apical pacing. Am J Cardiol. 2010;105:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace. 2012;14:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Lim HS. The prescription of minimal ventricular pacing. Pacing Clin Electrophysiol. 2012;35:1528-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Rosenblum AM. Marked interatrial and atrioventricular conduction delay with enhanced atrial-based managed ventricular pacing: electrocardiogram-echocardiogram Doppler correlation. Circulation. 2010;122:e494-e496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Vardas PE, Simantirakis EN, Kanoupakis EM. New developments in cardiac pacemakers. Circulation. 2013;127:2343-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nielsen JC, Thomsen PE, Højberg S, Møller M, Vesterlund T, Dalsgaard D, Mortensen LS, Nielsen T, Asklund M, Friis EV. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Mabo P, Cebron JP, Solnon A, Tassin A, Graindorge L, Gras D. Non-physiological increase of AV conduction time in sinus disease patients programmed in AAIR-based pacing mode. J Interv Card Electrophysiol. 2012;35:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Krzyżanowski K, Michałkiewicz D, Orski Z, Wierzbowski R, Ryczek R, Cwetsch A. Minimizing right ventricular pacing in patients with sinus node disease and prolonged PQ interval: the impact on exercise capacity. Cardiol J. 2014;May 20; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Verlato R, Botto GL, Massa R, Amellone C, Perucca A, Bongiorni MG, Bertaglia E, Ziacchi V, Piacenti M, Del Rosso A. Efficacy of low interatrial septum and right atrial appendage pacing for prevention of permanent atrial fibrillation in patients with sinus node disease: results from the electrophysiology-guided pacing site selection (EPASS) study. Circ Arrhythm Electrophysiol. 2011;4:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Olshansky B, Day JD, Lerew DR, Brown S, Stolen KQ. Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter-defibrillators. Heart Rhythm. 2007;4:886-891. [PubMed] |