Published online Feb 16, 2015. doi: 10.12998/wjcc.v3.i2.196
Peer-review started: June 10, 2014
First decision: July 18, 2014
Revised: October 27, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: February 16, 2015
Processing time: 241 Days and 11.8 Hours
The differentiation syndrome is an inflammatory reaction with increased capillary permeability that occurs in up to 25% of patients with acute promyelocytic leukemia treated with all-trans retinoic acid. A 50-year-old man with acute promyelocytic leukemia underwent chemotherapy with idarubicin and all-trans retinoic acid. On day +21 the patient developed pruritic prepatelar papules as well as several 10 mm subcutaneous nodules in both thighs accompanied by persistent fever. On the day +25 the patient presented with bilateral pulmonary crackles, infiltrates in the right lower lobe and severe hypotension which required dopamine infusion. Biopsy of one of the thighs nodules was performed. A Sweet syndrome associated to a differentiation syndrome was suspected. All-trans retinoic acid therapy was discontinued and dexamethasone was administered. In 48 h the patient showed remission of the fever and the infiltrates and the skin lesions acquired a residual aspect. It is debatable whether these two syndromes are distinct entities with common mechanisms or whether they are poles of the same spectrum. Dermatologists and hematologists must be aware of these two syndromes and its pathophysiologic association.
Core tip: It is debatable whether the differentiation syndrome and the sweet syndrome are distinct syndromes with common mechanisms or whether they are poles of the same spectrum. We believe that there may be more cases of differentiation presenting with skin sweet syndrome lesions, which are underdiagnosed, overshadowed by the critical state of these patients. Dermatologists and hematologists must be aware of these two syndromes and its pathophysiologic association. It is very likely that these two specialties are staring the same phenomenon from two different points of view.
- Citation: Solano-López G, Llamas-Velasco M, Concha-Garzón MJ, Daudén E. Sweet syndrome and differentiation syndrome in a patient with acute promyelocytic leukemia. World J Clin Cases 2015; 3(2): 196-198
- URL: https://www.wjgnet.com/2307-8960/full/v3/i2/196.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i2.196
All-trans retinoic acid (ATRA) therapy induces the differentiation of the myelogenous leukemic cells in acute promyelocytic leukemia (APL). The differentiation syndrome (DS) is an inflammatory reaction with increased capillary permeability that occurs in up to 25% of patients with APL treated with ATRA. It is characterized by respiratory distress, fever, pulmonary infiltrates, pleuropericardic effusions, renal failure and hypotension, with a mortality of up to 30% without treatment[1]. The association of sweet syndrome (SS) and DS has been exceptionally described in the literature.
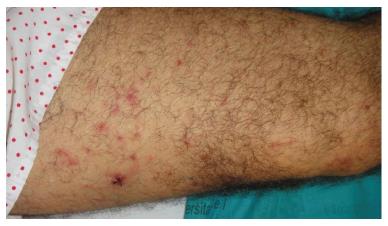
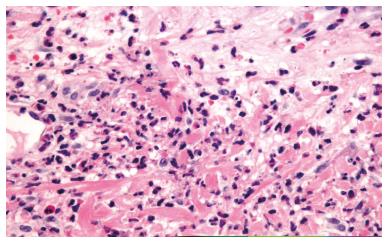
A 50-year-old man presented with pancytopenia on a routinal analysis. A bone marrow (BM) aspirate showed 73% of blasts; homogeneous medium to large cells with visible nucleoli in most cases and clasmatosis. Auer rods were also seen. The red series was decreased without megakaryocytes. The BM biopsy showed that the hematopoietic parenchyma was replaced by a proliferation of myeloid cells showing a monomorphic appearance. The neoplastic cells were positive for myeloperoxidase and CD117 and negative for CD34, TdT and Glycophorin. Thirty percent of BM cells were positive for PML-RARa by fluorescence in situ hybridization (FISH). Diagnosed of APL the patient underwent chemotherapy with idarubicin 12 mg/m2 and ATRA 45 mg/m2. On day +21 of ATRA therapy, the patient developed pruritic erythematous 3-5 mm prepatelar papules as well as several 10 mm subcutaneous nodules in both thighs along with persistent fever (Figure 1). On day +25, the patient presented with bilateral pulmonary crackles, infiltrates in the right lower lobe and severe hypotension which required dopamine infusion. No features of disseminated vascular coagulation were present. The patient did not gain or lose weight and no renal or hepatic dysfunction was observed. Empirical antibiotic and antifungical treatment was started. A cutaneous biopsy of one papule showed moderate edema in the papillary dermis with perivascular infiltrates consisting predominantly of confluent neutrophils, without vasculitis or involvement of the adipose tissue (Figure 2). Blood, urine and biopsy cultures were negative. Skin lesions occurred along with the neutrophil count recovery and the disappearance of the promyelocytes in the BM smears. Based on the data, a diagnosis of SS associated with DS was made. ATRA therapy was discontinued and dexamethasone 10 mg every 12 h was administered. In 48 h the patient showed remission of the fever and the pulmonary infiltrates and skin lesions cleared. On day +29, a new BM aspiration FISH study did not show the PML-RARa translocation.
Haematologists reintroduced ATRA as maintenance therapy for the APL along with corticosteroids without new recurrences.
Cases of drug-induced SS associated with ATRA has been exceptionally described in the literature. Although systemic manifestations in SS are unco-mmon, there are cases of biopsy proven pulmonary involvement[2-4].
As far as we are concerned, there are only 2 cases of SS associated with DS in patients with APL (Table 1)[2,5].
| Ref. | Age(yr)/sex | Cutaneous location | Biopsy | Onset of skin lesions after ATRA induction therapy (d) | Onset of the DS after ATRA induction therapy (d) | DS signs and symptoms | Steroid response | Time until improvement |
| Takada et al[5] | 49/F | Arms | Yes | 18 | 28 | Respiratory distress | Yes | 24 h |
| Astudillo et al[2] | 46/M | Trunk, arms, lower extremities | Yes | 6 | uk | Weight gain | Yes | Unknown |
| This case | 50/M | Trunk lower extremities | Yes | 21 | 25 | Respiratory distress Hypotension | Yes | 48 h |
It is debatable whether the DS and the SS are distinct syndromes with common mechanisms or whether they are poles of the same spectrum. They share common features such as fever, infiltration of neutrophils and improvement with steroid therapy. One of the differences between these two syndromes is that in most cases of SS, the involvement is limited to the skin while the main difference is the capillary leakage in the DS which is produced by the cytokine storm released by the promyelocytes as they mature. ATRA induces the differentiation of myelogenous leukemic cells into mature myeloid cells conferring them functional properties with modification of their migratory capability.
We know that these two syndromes are caused by ATRA therapy but we cannot rule out the possibility that they can be the sides of the same phenomenon with common mechanisms. For some authors, the SS and the DS are different inflammatory reactions with common mechanisms induced by ATRA therapy[6] while Ueno et al[7] thought that the SS due to ATRA therapy could represent a partial form of the DS.
We believe that there may be more cases of DS presenting with skin SS lesions which are underdiagnosed, overshadowed by the critical state of these patients. Dermatologists and hematologists must be aware of these two syndromes and its pathophysiologic association. It is very likely that these two specialties are staring the same phenomenon from two different points of view.
A 50-year-old man with acute promyelocytic leukemia presented with erythematous prepatelar papules as well as several subcutaneous nodules in both thighs along with persistent fever, pulmonary crackles and hypotension.
Sweet syndrome lesions and systemic symptoms in a patient who underwent chemotherapy with idarubicin and all-trans retinoic acid (ATRA).
Sepsis, drug reaction.
Blood, urine and biopsy cultures were negative.
Pulmonary infiltrates in the right lower lobe on a chest radiography.
A biopsy of a papule showed moderate edema in the dermis with perivascular infiltrates consisting predominantly of confluent neutrophils, without vasculitis or involvement of the adipose tissue.
Dopamine infusión, empirical antibiotics, ATRA therapy discontinued and dexamethasone 10 mg every 12 h.
As far as the author are concerned, there are only 2 cases of Sweet syndrome associated with differentiation syndrome in patients with acute promyelocytic leukemia.
Sweet syndrome is an inflammatory neutrophilic skin condition characterized by a sterile infiltrate of normal polymorphonuclear leukocytes.
The authors believe that there may be more cases of differentiation syndrome presenting with skin sweet syndrome lesions which are underdiagnosed, overshadowed by the critical state of these patients.
In the submitted manuscript the authors described a rare disorder–a drug-induced sweet syndrome associated with ATRA therapy for a patient with acute promyelocytic leukemia (APL). The association of sweet syndrome and ATRA-induced differentiation syndrome is rarely observed in APL. The case in this report is well-described, along with relevant lab results.
P- Reviewer: Dovat S, Sharma P S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3:e2011059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Astudillo L, Loche F, Reynish W, Rigal-Huguet F, Lamant L, Pris J. Sweet’s syndrome associated with retinoic acid syndrome in a patient with promyelocytic leukemia. Ann Hematol. 2002;81:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Astudillo L, Sailler L, Launay F, Josse AG, Lamant L, Couret B, Arlet-Suau E. Pulmonary involvement in Sweet’s syndrome: a case report and review of the literature. Int J Dermatol. 2006;45:677-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Cohen PR. Sweet’s syndrome--a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 530] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 5. | Takada S, Matumoto K, Sakura T, Shiozaki H, Miyawaki S. Sweet’s syndrome followed by retinoic acid syndrome during the treatment of acute promyelocytic leukemia with all-trans retinoic acid. Int J Hematol. 1999;70:26-29. [PubMed] |
| 6. | Shirono K, Kiyofuji C, Tsuda H. Sweet’s syndrome in a patient with acute promyelocytic leukemia during treatment with all-trans retinoic acid. Int J Hematol. 1995;62:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Ueno R, Takeuchi J, Shimizu T, Kumagai T, Sawada U, Horie T. [Development of Sweet’s syndrome during all-trans retinoic acid therapy for acute promyelocytic leukemia]. Rinsho Ketsueki. 2000;41:718-722. [PubMed] |