INTRODUCTION
Cases with solitary brain space occupying lesions (e.g., tumor, intracranial infection/abscess, granuloma, neurocystocercosis, tuberculoma, multiple sclerosis) may pose a diagnostic difficulty particularly when brain biopsy can’t be done due to deep location of the lesion. For such cases, clinicians often start corticosteroids to reduce manifestations of increased intracranial pressure (ICP) (caused by the intracranial mass and the surrounding vasogenic edema) and to give brain radiation if there is a high suspension for the presence of malignant lesion[1,2]. Also such patients should be routinely followed by magnetic resonance imaging (MRI) even after the disappearance of the enhancing lesion for at least 3-5 years[3]. The disappearance or decrease of the initial brain space occupying lesion (SOL) volume to ≤ 70% either spontaneously or after steroid treatment before establishing the definitive diagnosis, has been referred as a vanishing brain lesion[4].
In the last decade, magnetic resonance spectroscopy (MRS) has been considered as a diagnostic test which helped to distinguish normal from abnormal brain tissue. Proton (1H) MRS measures some unique tissue metabolites which provide valuable information regarding the severity of the brain lesion, pathogenesis, prognosis and response to therapy. Briefly, in MRS, peaks which are proportional to the concentration of the given metabolite, are arranged along a flat baseline according to their radiofrequency (measured in units called parts per million or ppm)[5]. The most commonly defined brain metabolites (1H-MRS spectrum) in which their patterns can be observed and correlate with different types of lesions (from right to left) include: free lipids (Lip), lactate (Lac), N-acetyl-aspartate (NAA), glutamate/glutamine (Glx), creatine (Cr)/phosphocreatine (the Cr peak), choline (Cho; the Cho peak), and myo-inositol (mI). In spectra obtained at long echo time (≤ 135 ms), the peaks for NAA, Cr, Cho, and lactic acid are prominent and sharp. They are also detected and quantified at short echo time (≥ 30 ms) MRS. In contrast, Lip, Glx and mI signals are detected only in short echo time MRS. In normal brain, NAA is synthesized in neurons, diffuses along axons and broken down in oligodendrocytes. In MRS, NAA is a marker of intact number of neurons in gray matter and the density of intact axons in white matter. The most prominent peak of NAA in MRS is the resonance at 2.0 ppm and it has concentration of 7.9-16.6 mmol/kg. NAA is a non-specific marker as its value is reduced in any disease associated with neuronal or axonal loss[6]. Cr is present at higher concentrations in the glia and it is a marker of brain energy. The most prominent peak of Cr in MRS is the resonance at 3.0 ppm and concentration of 5.1-10.6 mmol/kg[7]. Cho is a marker of brain injury of non-specific type. Cho level reflects the brain membrane metabolism with cellular turnover. The most prominent peak of Cho in MRS is the resonance at 3.2 ppm and concentration of 0.9-2.5 mmol/kg[6]. Lipids comprise about 20% of brain weight. Lipids are normally absent from 1H spectrum, and its appearance at 0.9-1.4 ppm resonances indicates presence of necrotic tissue (i.e., breakdown of cell membrane and release of fatty acids)[8]. The mI is a marker of glial proliferation. It resonates at 3.6 ppm and concentration of 3.8-8.1 mmol/kg[9]. The glutamate/glutamine peak represents a mixture of excitatory and inhibitory brain neurotransmitters. Glutamate is mainly stored in neurons whereas glutamine concentration is higher in astrocytes. Both, Glx have two groups of resonances, the first group resonances at 3.6-3.9 ppm whereas the second group resonances at 2.0-2.6 ppm and concentration of 6.0-12.5 mmol/kg for glutamate and 3.0-5.8 mmol/kg for glutamine. Excess glutamate in active lesions could contribute to axonal damage, brain atrophy and neurological disability[10].
Malignant brain tumors are differentiated from other focal lesions, including multiple sclerosis (MS), radiation necrosis and infections/abscess by absent NAA, excess Cho and lip. Spectra with elevated Cho, a Cho/Cr index greater than 1.3 and diminished NAA levels are associated with aggressive neoplasms (e.g., malignant lymphoma and glioma). Tumor recurrence is characterized by high Cho/Cr and Cho/NAA ratios. A multi-voxel Cho/Cr ratio of > 1.54 and Cho/NAA ratio of > 1.05 was found to have 93.1% and 89.7% accuracies for diagnosis of tumor recurrence, respectively[11,12]. In MS, the abnormal increases in total Cr, total Cho, mI, Lac, lipids and macromolecules are markers for acute demyelination in MS. NAA is reduced in acute MS lesions and in normal appearing white matter, even distant to acute and chronic-lesions. Increased NAA to subnormal values occurs during remyelination. mI is increased in chronic MS (a marker for astrocytic gliosis). Reduced NAA peak represents neuronal/axonal dysfunction or loss. Elevated Cho peak represents enhanced cell-membrane turnover and is seen in demyelination, remyelination, inflammation, or gliosis[13]. In radiation necrosis (defined as a death of normal brain tissue caused by radiation therapy), in which the pathological features include progressive cellular necrosis (coagulative necrosis), inflammatory changes and reactive glial cell proliferation and gliosis, MRS shows elevated Cho, mI, lactate and lipid peaks[14].
This paper described a child with a symptomatic parenchymal brain mass but showed marked clinical recovery and disappearance of the original brain mass shortly after starting treatment. In this case report, the phenomenon of a tumor/or pseudo-tumor remission and the problems related to its differential diagnosis and treatments were discussed. The significance of using MRS to distinguish neoplastic from non-neoplastic nature of the intra-axial lesion in our patient was also discussed.
CASE REPORT
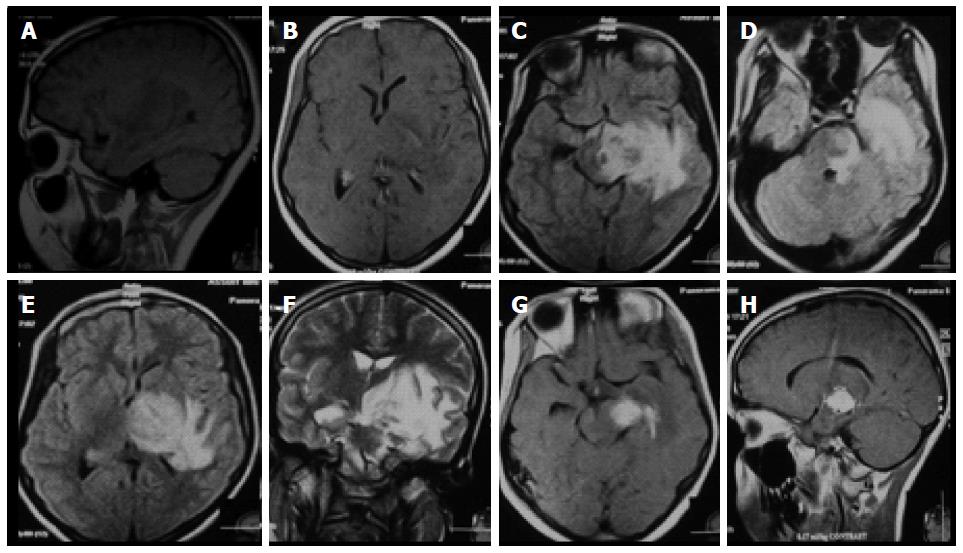
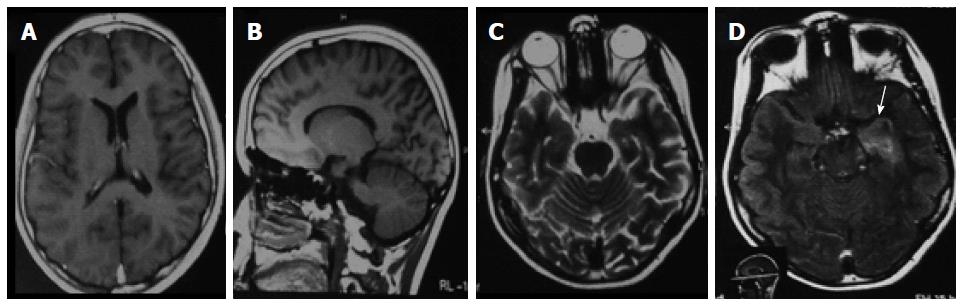
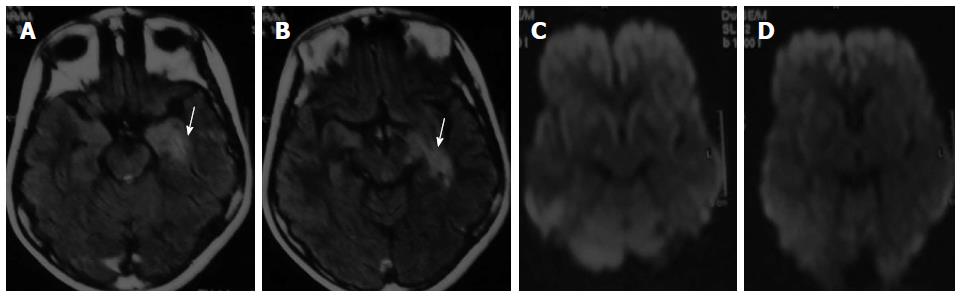
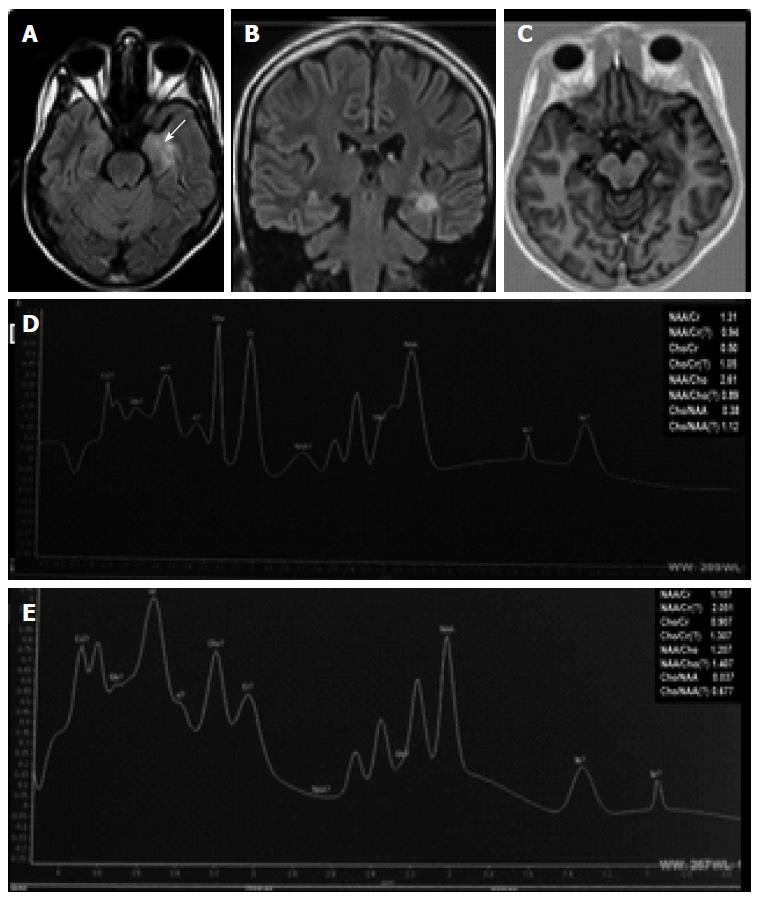
At September 2009, a 14-year-old child presented with a history of fever, anorexia, generalized body ache, loss of weight and headache since two months, which progressed to repeated vomiting, nausea, lethargy and blurring of vision in the last month. Prior to neurologic consultation, the patient was admitted to a fever hospital for one week because of the unexplained high grade fever. One year ago, the patient had past history of body aches and recurrent arthritis which was attributed to recurrent tonsillitis and based on the advice of the ear, nose and throat (ENT) physician, the patient did tonsillectomy. The mother said that although it was expected that the patient will be better after tonsillectomy but unfortunately, he had recurrent fever and generalized body aches till the time of presentation. On neurological examination, the child was feverish and looked toxic. He was alert, oriented and his higher mental functions testing were normal. He had right hemiparesis and right upper motor neuron facial paralysis. His fundus examination was normal. MRI-brain [1.5-Tesla, standard (T1W) pre- and post-contrast, T2W, fluid attenuated inversion recovery (FLAIR) brain imaging] revealed presence of a large intra-axial mass (25 mm × 19 mm) in the left temporal lobe with extension to the adjacent brainstem. It showed hypointense signal in T1W, hyperintense signals in T2W and FLAIR images and homogenous enhancement with gadolinium (Gd). It was surrounded by moderate perifocal vasogenic edema with mass effect in the form of compression of the third ventricle with midline shift (Figure 1). The clinical and radiological findings were highly suggestive of a neoplastic lesion (malignant lymphoma or glioma). The patient was examined for lymphadenopathy and organomegaly. He underwent laboratory workup [for complete blood count, erythrocyte sedimentation rate (ESR), glucose, electrolytes, lactic dehydrogenase (LDH), liver and renal functions], abdominal ultrasonography and chest radiographs to rule out the presence of systemic lymphoma but no bone marrow evaluation was done. Blood tests revealed leukocytosis (16000 cells/μL) and elevated ESR (30/52). In view of the presence of fever, manifestations of increased intracranial pressure (ICP) and the prominent cerebral edema associated with the intracranial lesion; intravenous antibiotics (cefotax 1 g/12 h per 7 d), mannitol (2 g/12 h per 48 h) and dexamethasone (8 mg/12 h) were initiated. The oncologist recommended whole craniospinal irradiation (brain = 4000 CGy/20 settings per 4 wk; spine = 2600CGy/13 settings per 3 wk) which was started 10 d after presentation. Within 15 d and even before the start of radiotherapy, the patient exhibited marked clinical recovery (up to complete improvement) but he developed subjective cognitive deterioration as a side effect of radiotherapy which was recovered after its discontinuation. Tapering of steroids was done over the next 4 mo. Follow up of the patient was done every 3 mo. The follow up MRI after 3 mo from onset showed disappearance of the original mass but presence of small lesion with hypointense signal in T1W and hyperintense in T2W and FLAIR signals in the antero-medial part of the left temporal lobe but did not show enhancement with Gd. At further follow up (September 2010) the patient condition was unremarkable and his MRI had the same small non-enhanced lesion (Figure 2). At August 2012, the patient developed recurrent generalization tonic-clonic convulsions. His EEG showed left temporal focus of epileptic activity. His MRI had the same small non-enhanced lesion (as that of 2010) with no restricted diffusion in diffusion weighted images (DWI) (Figure 3). The seizures frequency was reduced with carbamazepine therapy (300 mg/12 h). At October 2014, his follow up MRI had the same small non-enhanced lesion (as that of 2010 and 2012) with no restricted diffusion in DWI. Single voxel proton (1H) spectroscopy at long and short echo times showed reduced values of choline to creatine (Cho/Cr: long ET = 0.05; short ET = 0.907), N-acetyl-aspartate to creatine (NAA/Cr: long ET = 1.31; short ET = 1.107) and N-acetyl-aspartate to choline (NAA/Cho: long ET = 0.037; short ET = 0.38) ratios which confirmed the absence of neoplastic activity but highly suggestive of gliotic lesion (Figure 4).
Figure 1 Cranial magnetic resonance imaging brain (on admission at September 2009) showing (A, B) sagittal and axial T1-weighted views with a solitary hypointense lesion in the left temporal lobe; (C-E) axial fluid attenuated inversion recovery and T2-weighted (F) images showing hyperintense lesion in the left temporal lobe encroaching on the adjacent brainstem with perifocal edema and mass effect; (G, H) axial and sagittal T1-weighted views showing homogenous solitary enhanced lesion in the left temporal lobe encroaching on the adjacent brainstem and surrounded by a moderate hypointensity.
Figure 2 Cranial magnetic resonance imaging brain (September 2010) showing (A, B) normal axial and sagittal TIW and (C) axial T2W images but (D) hyperintense lesion in the antero-medial region of the left temporal lobe (white arrow) in fluid attenuated inversion recovery image.
Figure 3 Cranial magnetic resonance imaging brain (August 2012) showing (A, B) hyperintense lesion in the antero-medial region of the left temporal lobe (white arrow) in axial fluid attenuated inversion recovery images (white arrow) and (C, D) normal diffusion weighted axial images.
Figure 4 Cranial magnetic resonance imaging and magnetic resonance spectroscopy (MRS) brain (October 2014) showing (A, B) hyperintense lesion in the antero-medial region of the left temporal lobe in axial and coronal fluid attenuated inversion recovery images (white arrow); and (C) no restricted diffusion in axial diffusion weighted axial images; (D, E) long and short time echo MRS showing reduced values of Cho/Cr (long ET = 0.
05; short ET = 0.907), NAA/Cr (long ET = 1.31; short ET = 1.107) and NAA/Cho (long ET = 0.037; short ET = 0.38) ratios. NAA: N-acetyl-aspartate; Cr: Creatine; Cho: Choline.
This study was conducted according to the principles established in Helsinki and approved by Assiut University Hospital ethics committee. Informed written consent was obtained from the patient and his parents to publish the details of his clinical history, laboratory and imaging data.
DISCUSSION
This case is significant as the parenchymal brain mass could not be distinguished from a neoplastic space occupying lesion (e.g., glioma or lymphoma) at presentation. Brain biopsy was not done due to deep location of the lesion. Complete clinical improvement was observed within 15 d on medical treatment including steroids and even before the start of radiotherapy. Corticosteroids were used empirically to reduce the manifestations of increased ICP and improve the surrounding vasogenic edema caused by the intracranial mass. The decision to give whole craniospinal irradiation was based on the high suspicion of a neoplastic lesion (e.g., lymphoma or glioma). Marked reduction of the intracranial mass with disappearance of enhancement was observed in MRI within 3 mo of the onset. Follow up of the patients up to 5 years showed absence of recurrence of the original lesion.
Based on the above findings, the suggestive differential diagnosis of the vanishing space occupying lesion in this child at presentation include: (1) tumor [e.g., primary central nervous system lymphoma (PCNSL) or glioma]; (2) tumor like demyelinating lesion or tumefactive multiple sclerosis (TMS); in clinical practice, most vanishing brain masses are frequently diagnosed as malignant tumors or multiple sclerosis (MS); and (3) intracranial infection/abscess/granulomas or tuberculoma.
For our patient, earlier at presentation, PCNSL was suggested. In patients presented with unclear intra-axial brain masses which regress with steroids, the diagnosis of PCNSL has to be considered[15]. PCNSL is a rare extranodal non-Hodgkin’s lymphoma[16]. PCNSL represents approximately 3%-4% of newly diagnosed central nervous system (CNS) tumors[17]. The typical MRI features of PCNSL include the presence of intra-axial single or multiple masses adjacent to cerebrospinal fluid space (CSF) with intermediate- to low-signal-intensity in T1W images and hypointense signal relative to the gray matter on T2W images, surrounding vasogenic edema, mass effect, restricted diffusion in DWI and intense homogenous enhancement with Gd[18,19]. Although, PCNSL is extremely rare in children and immunocompetent individuals[20,21], we suggested the diagnosis of PCNSL based on the MRI appearance of large solitary deep hemispheric infiltrative lesion[22] and rapid remission with steroid even before the start of radiotherapy. However, PCNSL is a malignant neoplasm and never considered as a self-limiting and recurrence is common within 18 mo. No cases have been reported yet for malignant brain tumors that recurred more than 5 years after spontaneous regression[23]. For our patient, the lacks of recurrence on follow ups for more than 5 years making such diagnosis less likely. This was also confirmed by the findings of MRS which will be discussed in the following section.
Also for our patient, the presence of fever prior to presentation and rapid remission with IV antibiotics and steroid suggest the diagnosis of TMS[24] or abscess/granuloma[25] but not the diagnosis of tuberculoma[26]. TMS is defined as a solitary large intracranial lesion larger than 2.0 cm in diameter associated with perilesional edema and mass effect[24]. TMS represents 1-2/1000 of cases of MS. TMS has been reported to be extremely rare in children in comparison to tumors and abscesses[27]. Diagnosis of MS depends on combination of clinical, neurophysiological, elevation of CSF immunoglobulin G (IgG) index and oligoclonal bands, and MRI of the brain and spine. Immunosuppressants (including steroids) and immunomodulators are the main therapies of TMS[28]. TMS lesion usually appears as open-ring (directed toward the cortical surface or to the basal ganglia) or closed ring or has diffuse, homogeneous, punctate, or concentric enhancement with Gd[29]. Although, CSF examination and gadolinium-enhanced MRI scan should differentiate between the MS and PCNSL, however, CSF may also be normal in fulminant conditions and short duration of the disease[30].
Furthermore, for our patient inflammatory pseudotumors or non-neoplastic lesions (e.g., abscess/granulomas) of unknown etiology and respone to steroids was also suggested[25]. Patients with intracranial infection/abscess/granulomas commonly have history of risk factors (e.g., immunocompromised state, dental, pulmonary or ear abscesses and intravenous drug use), fever, abnormal labs (as high erythrocytic sedemintation rate or C-reactive protein) and abnormal CSF suggesting CNS infection. Presentation is usually of acute onset with manifestations of increased ICP, seizures and focal neurological deficits. MRI-brain of brain abscess usually shows ring enhancement which is often complete with regular margin[31].
Magnetic resonance spectroscopy (MRS) was not done to the patient at presentation (2009) to distinguish neoplastic from non-neoplastic nature of the mass due to lack of availability. However and fortunately, it was available later and was done to the patient when he developed epilepsy (2012). For our patient, the focal lesion in the left antero-medial region of the temporal lobe found in the MRI (2010-2014) is the cause of the patients’ left temporal lobe epilepsy with secondary generalization. The suggested differential diagnosis of the lesion according to the conventional MRI include: (1) tumor recurrence; (2) radiation necrosis; (3) multiple sclerosis; and (4) post-infective/inflammatory gliosis. MRS helped to distinguish tissue changes due to different brain lesions as discussed below.
For our patient, the reduced Cho/Cr (short ET = 0.907; long ET = 0.05), NAA/Cr (short ET = 1.107; long ET = 1.31) and NAA/Cho (short ET = 0.38; long ET = 0.037) ratios confirm the absence of tumor recurrence. Furthermore, the lack of reduced diffusion in DWI also confirms the absence of tumor recurrence[32].
For our patient, the diagnosis of radiation necrosis was suggested based on the facts that children are more susceptible to radiation necrosis than adults[33] and it usually occurs approximately 2-32 mo after radiotherapy, with 85% of cases occurring within 2 years. Delayed radiation-induced brain injury is a relatively common complication of radiation therapy representing 3%-24%[34]. Radiation necrosis is usually presented as a solitary periventricular white matter lesion, because of excess oligodendrocytes in these areas and a poor blood supply that produces ischemia[35]. The typical MR appearance of radiation necrosis is a soap bubble or Swiss cheese-like enhancing periventricular mass[36-38] and elevated Cho, mI, lactate and lipid peaks in MRS[39,40]. Radiation necrosis is related to both the volume of irradiated brain and the total administered radiation dose[41]. It has been reported radiation necrosis is extremely rare (5%) at doses < 45 Gy given over 25 fractions, or when the fractional dose is < 2 Gy/d but often occurs with total doses of > 60-70 Gy[34] or when the fractional dose is ≥ 2 Gy/d. Our patient was given whole brain radiotherapy in a dose of 4000 CGy/20 settings per 4 wk (i.e., < 2 Gy/d) making the diagnosis of radiation necrosis less likely. Also the absence of enhancement of the new lesion further confirms that the lesion in our patient is not a radiation necrosis.
Although, the results of MRS of our patients may suggest remyelination and gliosis following TMS, however, the presentation with seizures and lack of relapse with enhanced lesions after 5 years of follow up makes the diagnosis of MS less likely[13].
For our patient, the reduced values of Cho/Cr, NAA/Cr and NAA/Cho and lack of enhancement of the lesion are consistent with the diagnosis of gliosis. Gliosis is the process of scarring in the central nervous system[42]. It results from the proliferation of glial cells or in a damaged brain tissue. It represents a healing process of brain injury whatever its nature. When neurons are injured, astrocytes proliferate in the region and manufacture glial-fibrillary acidic protein. This compound causes the astroglia to form a dense and fibrous tissue: The glial scar. Gliosis can take from a few days to many months to reach its final form. Gliosis is diagnosed by immunohistochemistry or MRI[43]. Gliosis occurred as a result of an acquired brain injury (most likely abscess, granuloma, inflammation) and it is the cause of temporal lobe epilepsy. Cr and Cho are glial markers. Gliosis typically presents with reduced levels of Cho, NAA, and Cr and observed lip peaks. Moderate levels of Cho and/or a Cho/Cr index < 1.3 are frequent with gliosis. This is supported by the followings: (1) the development of epilepsy in our patient occurred as a result of a focal lesion in the antero-medial region of the left temporal lobe which is suggestive of gliosis with no evidence of neoplastic activity as confirmed by MRS; and (2) mesial temporal lobe epilepsy (due to hippocampal sclerosis) is characterized by hippocampal atrophy, decreased NAA, and a low NAA/Cr ratio which are attributed to neuron loss and gliosis[39].
ACKNOWLEDGMENTS
I would like to thank the patient’s parents for their cooperation and providing approval to publish the clinical, laboratory and imaging results of this case presentation.
COMMENTS
Case characteristics
A 14-year-old child with history of acute manifestations of increased intracranial pressure and right sided hemiparesis which improved completely within two weeks and followed after 3 years by epilepsy.
Clinical diagnosis
The patient was having right sided hemiparesis due to brain space occupying lesion which was complicated by recurrent generalized epilepsy.
Differential diagnosis
Brain tumor (e.g., primary central nervous system lymphoma or glioma); tumor like demyelinating lesion or tumefactive multiple sclerosis and intracranial infection or abscess/granulomas.
Laboratory diagnosis
Blood tests revealed mild leukocytosis and elevated erythrocyte sedimentation rate (ESR).
Imaging diagnosis
Initially at presentation, magnetic resonance imaging-brain showed a large intra-axial mass (25 mm x 19 mm) in the left temporal lobe with hypointense signal in T1W, hyperintense signals in T2W and fluid attenuated inversion recovery (FLAIR) images and homogenous enhancement with Gd suggesting neoplastic lesion (malignant lymphoma or glioma) while follow up after 5 years, MRI showed small non-enhanced lesion in the antero-medial part of the left temporal lobe with hypointense signal in T1W and hyperintense in T2W and FLAIR signal and reduced choline (Cho)/creatine (Cr), N-acetyl-aspartate (NAA)/Cr and NAA/Cho ratios in magnetic resonance spectroscopy (MRS) which confirmed absence of neoplastic activity but suggestive of gliosis.
Pathological diagnosis
Inflammatory brain space occupying lesion complicated by gliotic lesion in the antero-medial part of the left temporal lobe.
Treatment
Brain dehydrating measures, antibiotics, corticosteroids and a course of craniospinal irradiation.
Related reports
In clinical practice; a vanishing brain space occupying lesion is commonly diagnosed as a neoplasm (e.g., lymphoma) or multiple sclerosis.
Term explanation
A vanishing brain space occupying lesion is defined as reduction or disappearance of a brain lesion spontaneously or after steroid treatment to ≤ 70% of its size before establishing its definitive diagnosis.
Experiences and lessons
In clinical practice, neuroimaging [including MRS or magnetic resonance imaging (MRI)] has to be done every 6-12 mo for at least 3-5 years to follow up after complete remission of the patient with a vanishing brain lesion.
Peer-review
The case report presents a vanishing brain space occupying lesion in a child over 5 years course of recovery and MRI follow-up. The prognosis was fortunately better. Text is well wrote and easily comprehensible with clear figures. The authors discussed the potential differential diagnosis, and recommended that MRS may be helpful to identify a potential vanishing brain space occupying benign lesion from tumor lesion in clinic.
P- Reviewer: Amiri M, Jiang B S- Editor: Tian YL L- Editor: A E- Editor: Wang CH