Published online Oct 16, 2015. doi: 10.12998/wjcc.v3.i10.894
Peer-review started: April 14, 2015
First decision: June 3, 2015
Revised: June 21, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: October 16, 2015
Processing time: 185 Days and 14.2 Hours
Primary splenic lesions are rare entities among which littoral cell angioma (LCA) is a recently described, uncommon vascular lesion that is unique to the spleen. It has heretofore been described primarily in pathologic series and has been found mostly to behave as a benign entity. A few reports of malignant variants have been reported. We present a case report of a solitary LCA discovered after splenectomy for an incidentally discovered splenic lesion, along with a literature review.
Core tip: Littoral cell angioma (LCA) is a rare benign vascular lesion of the spleen. LCA can range from no symptoms to a vague set of symptoms such as: abdominal pain, splenomegaly, thrombocytopenia, anemia, fever, chills, weakness and fatigue. Diagnosis is made by histopathology after splenectomy.
- Citation: Bailey A, Vos J, Cardinal J. Littoral cell angioma: A case report. World J Clin Cases 2015; 3(10): 894-899
- URL: https://www.wjgnet.com/2307-8960/full/v3/i10/894.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i10.894
Primary splenic tumors are uncommon and are classified as lymphoid tumors, non-lymphoid tumors, and tumor like lesions[1-12] (Table 1). Among non-lymphoid tumors, vascular neoplasms are the most common and arise from the vascular elements that compose the splenic red pulp. Conversely, the lymphatic tissue containing splenic white pulp is from where lymphoid neoplasms arise. In regards to vascular tumors of the spleen, the biologic behavior can be both benign and malignant.
| Category | Type | Clinical | Pathological | Radiological |
| Non Hodgkin lymphoma | Fevers, sweats, change in weight are common symptoms | Derived from B or T cells, lympho-proliferative | CT: Hypodense nodules, diffuse or military distribution | |
| MRI: Isotense on precontrast images, hypotense on postcontrast images | ||||
| Lymphoid | Hodgkins lymphoma | Spleen is a rare primary site | Nodular sclerosis subtype, Reed-Sternberg cells | CT: Hypodense nodules with nodular sclerosis |
| Inflammatory pseudotumor | Secondary to inflammatory response to infection or injury Benign | Spindle cells, lymphocytes in fibroblastic stroma | CT: Well circumscribed +/- calcifications, hypoattenuating | |
| MRI: Hypo- or isointense on T1 images. Variable signaling on T2 images | ||||
| Plasmacytoma | Rare diagnosis | Diffuse infiltration of plasma cells | Not well categorized findings | |
| Histocytic lymphoma | Non specific symptoms, elevated ESR | Nodules with central necrosis | US: Cystic appearance | |
| CT: Sharply demarcated with central necrosis | ||||
| Hemangioma | Benign, slow growth, asymptomatic | Sinusoidal epithelium, proliferation of vascular channels | Solid to cystic components | |
| US: Echogenic solid to complex mass | ||||
| CT: Iso- to hypoattenuation associated with calcification | ||||
| MRI: Hypo- to isointense on T1 images, hyperintense on T2 images | ||||
| Hamartoma | Benign, asymptomatic. Associated with tuberous sclerosis and Wiskott Aldrich | Solid nodules, well circumscribed, well defined gross appearance. Unorganized vascular channels with fibrotic cords | US: More sensitive than CT, solid mass +/- calcification | |
| CT: Isoattenuating | ||||
| MRI: Isointense on T1 images, hyperintense | ||||
| Lymphangioma | Asymptomatic, benign, mostly in children | Multiple solitary nodules, Flattened endothelium with proteinaceous material in a capillary, cavernous or cystic presentation | US: Splenic cysts hypoechoic septations | |
| CT: Thin walled low attenuation masses, subcapsular location | ||||
| MRI: Hypointense on T1 images, hyperintense on T2 images | ||||
| Vascular | Littoral cell angioma | Asymptomatic, benign with malignant potential | Well delineated nodules of anastomosing vascular channels with endothelial cells | US: Hypoechoic to hyperechoic |
| CT: Iso to hypoattenuating with contrast enhancement | ||||
| MRI: Low intensity lesions | ||||
| Angiosarcoma | Older patients, malignant, nonspecific symptoms | Diffuse involvement of spleen arises from sinus endothelial cells, high mitotic rate | US: Complex mass, heterogenous, necrotic degeneration | |
| CT: Ill-defined mass with heterogenous enhancement, punctate calcification | ||||
| MRI: Mixed signal intensity on T1 and T2 | ||||
| Hemangioendothelioma | Nonspecific symptoms, young adults | Variable morphologic appearance | US: Hypoechoic mass | |
| CT: Low attenuated mass with enhancement of solid portions | ||||
| MRI: Heterogenous solid mass. Hypointense on T1 and T2 images | ||||
| Fibrosarcoma | Asymptomatic | Well differentiated, spindle shaped, fibroblasts, collagen is commonly present | Non specific imaging findings | |
| Non- lymphoid | Lipoma | Asymptomatic | Adipose tissue, no atypia, cytoplasmic vacuoles | CT: Well defined fat density mass |
| Kaposi sarcoma | Associated with HIV/AIDS +/- skin lesions | Spindle cell proliferation, spongelike vascular channels | CT: Ill-defined nodules, homogeneous | |
| US: Hyperechoic nodules | ||||
| Peliosis | Associated with anabolic steroid, TB, AIDS, cancer. Asymptomatic | Cyst like blood filled cavities within splenic parenchyma | US: Echogenic mass | |
| CT: Hypoattenuating, multiloculated with septa | ||||
| Tumor like | Nonparasitic cysts | Congenital or neoplastic in origin. Benign. | Varies according to type of cyst including dermoid cyst | US: Cystic lesions with solid components |
| CT: Hypoattenuating lesions, well defined | ||||
| Granulomas | Associated with chronic granulomatous disease and sarcoidosis | Granulomas non-necrotizing or necrotizing | CT: Hypodense nodules | |
| MRI: Hypointense T1 and T2 |
Littoral cell angioma (LCA) of the spleen is a rare vascular tumor that was first described in 1991 by Bhatt et al[13]. Initially thought to be benign, the biologic behavior of LCA has not been firmly established, as there have been several reports of LCA with malignant features[14,15]. LCA may occur at any age and has no gender predilection. To date, a total of 110 cases have been reported in the literature with 4 published pathologic series and 3 published case series[13,16-32].
LCA is discovered as a splenic lesion in patients who are undergoing a workup for laboratory evidence of anemia or thrombocytopenia[33-36]. Imaging findings of LCA are nonspecific and splenomegaly, to a varying degree, is a common finding. Due to the nonspecific findings that often result from the diagnostic workup, splenectomy is often performed for both diagnostic and therapeutic purposes. In the present report, a case of an incidentally discovered LCA is described.
A 65-year-old female presented to the outpatient oncology surgery clinic for surgical evaluation of a 2.2 cm splenic lesion. The lesion was discovered incidentally on a computed tomography (CT) abdomen/pelvis study to evaluate recurrent urinary tract infections (Figure 1). Also, the CT scan revealed a second incidental finding of a 1.1 cm right adrenal nodule. The patient was asymptomatic without abdominal pain, persistent fever, chills, weight loss, or other constitutional symptoms. Her past medical history included hypertension, diabetes mellitus, gout and peripheral neuropathy. Physical examination was unremarkable except for abdominal wall scars from prior open hysterectomy, cholecystectomy and left nephrectomy, the latter of which which was performed at a young age for a nonfunctioning left kidney secondary to congenital ureteropelvic junction obstruction. A biochemical workup to exclude a functioning adrenal tumor was performed and included serum renin and aldosterone levels as well as 24 h urinary fractionated metanephrine and cortisol levels, all of which were within the limits of normal. Of note, she was not leukopenic, anemic or thrombocytopenic.
Given the size of her incidentally discovered splenic lesion, she was offered operative resection for diagnostic purposes. Based on her extensive prior surgical history, an open approach to the splenectomy was planned. The patient received preoperative pneumococcal, meningococcal and haemophilus B vaccinations. The operation and recovery were uneventful and the patient was discharged to home on postoperative day four.
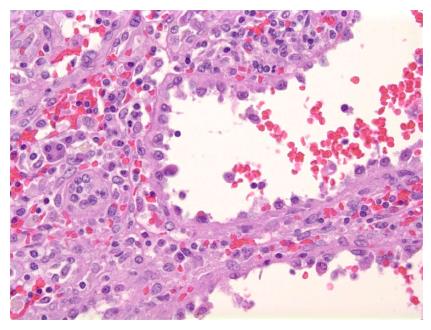
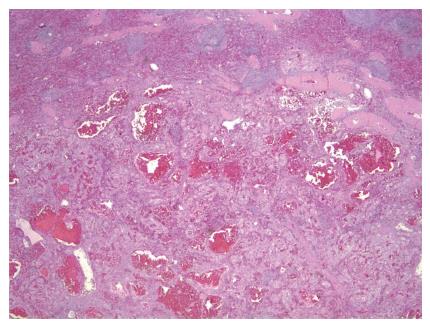
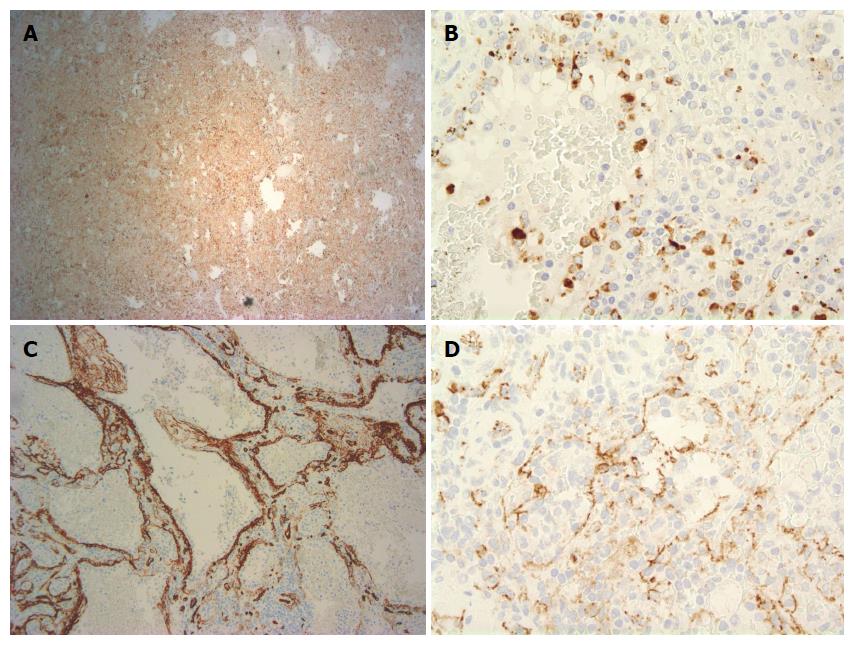
Grossly, the spleen weighed 270 g and measured 23.3 cm × 18.1 cm × 7.2 cm. The splenic lesion measured 2 cm × 2 cm × 2 cm. Histopathologically, the tumor was found to have anastomosing vascular channels with large cyst formations which were lined predominately by tall, histiocytoid cells which projected into the vascular spaces along with interspersed flat endothelial cells (Figures 2 and 3). Immunohistochemically, the cells compromising the tumor stained positive for CD68 and lysozyme (Figures 4A and B). The specimen also showed variable expression of S100. CD34 and CD31 stains were positive on the endothelial cells, however negative on the histiocytoid cells (Figures 4C and D). Final pathologic diagnosis was littoral cell angioma.
LCA is a rare vascular neoplasm of the spleen. It has been found to affect both men and women in an equal distribution. Given the relative lack of symptom specificity, LCA is most often found incidentally as a splenic mass on abdominal imaging; however, two cases of LCA presenting with splenic rupture and hemoperitoneum have been reported[37,38]. The sonographic appearance of LCA is variable, and ranges from a hypoechoic to a hyperechoic mass with a mottled texture[14]. On contrast enhanced CT, LCA is isodense to slightly hypodense as related to the surrounding splenic parenchyma in both the arterial and early portal venous phase[39,40]. Magnetic resonance imaging characteristically shows a T1 and T2 hypointense mass. LCA is often multifocal and lesions can be variable in size[14]. The differential diagnosis of lesions that can mimic LCA on imaging includes lymphangioma, hamartoma, lymphoma, Kaposi’s sarcoma, and hemangioma. Therefore, a definitive diagnosis can only be obtained pathologically[41].
Pathologically, LCA is a vascular tumor of the spleen that represents a tumoral counterpart of the normally present littoral cells that line the splenic sinus channels of the red pulp[30]. First described by Falk et al[33] in 1991 in a pathologic series of 17 cases, this new entity was described histologically as consisting of anastomosing vascular channels with cyst like spaces and papillary projections. The endothelial cells lining the channels are tall and plump compared to the flat endothelial cells lining the channels in a normal spleen. Immunohistochemically, LCA is characteristically CD 34 negative, CD 68 positive, CD 21 positive and CD 8 negative[22]. Additionally, the epithelial cells in LCA do occasionally express S-100 protein[16]. High expression of formin homology domain protein 1 (FHOD1) distinguished littoral cells from LCA. FHOD1 protein is expressed by normal littoral cells, not by LCA[42]. Further research has been done evaluating molecular markers and LCA to help aide in the accurate diagnosis of LCA tumors. O’Malley et al[43], looked at splenic lesions and the activity of the Ets Related Gene (ERG) and the Wilms Tumor-1 gene (WT-1). They found that LCA splenic lesions had a pattern of ERG positive and WT-1 negative[43]. Of the other types of splenic lesions evaluated cavernous hemangiomas were found to have the same pattern, therefore these markers are not specific enough alone to make the diagnosis of LCA.
LCA has most commonly been described as a benign process. However, observations of malignant behavior have been described[41]. In one case, metastatic lesions were found in the liver and retroperitoneum four years after splenectomy for LCA[44]. This case initially had symptoms of ureteral obstruction and renal failure. In comparison, our patient did not have ureteral obstruction however did have recurrent UTI’s and a history of congenital ureteropelvic junction obstruction. Kranzfelder et al[45], showed a case of familial individuals with LCA and primary splenic angiosarcoma, raising the question of possible malignant transformation. There were no similar signs and symptoms between their case and the presented case. Harmon et al[17], published a case report of a patient with transitional cell carcinoma of the bladder with suspected splenic metastasis. The pathology revealed LCA and not splenic metastasis. Ben-Izhak et al[14], showed a case of malignant littoral cell tumor naming it littoral cell hemangioendothelioma. This case report featured a symptomatic patient with liver metastasis eight years after splenectomy. In reviewing all of these cases, the immunohistochemical pattern was similar giving the diagnosis of LCA.
LCA has been shown to be rarely associated with visceral malignancies including colorectal adenocarcinoma, pancreatic cystadenocarcinoma, pancreatic neuroendocrine tumor, renal cell cancer, hepatocellular carcinoma, non-small cell lung cancer, seminoma, ovarian cystadenocarcinoma, papillary thyroid cancer and transitional cell carcinoma of the bladder[17]. Furthermore, there have been a few reports describing an association of LCA with immunological disorders, such as, ankylosing spondylitis, myelodysplastic syndrome, non-Hodgkin lymphoma, Crohn’s disease, Wiskott Aldrich syndrome, chronic glomerulonephritis, aplastic anemia and Gaucher’s disease[17,22,46]. Given the association of LCA with other malignancies as well as the few reported cases of malignant behavior, patients should undergo close follow up after splenectomy; however, no established postoperative surveillance guidelines exist.
Littoral cell angioma is a rare vascular tumor of the splenic red pulp, and is typically an incidental finding on abdominal imaging. The splenic lesion can only truly be differentiated from other splenic masses by histologic examination. Splenectomy is the appropriate treatment, as LCA has a variable behavior pattern of which malignant tendencies are worrisome. Furthermore, longitudinal surveillance in the postoperative phase is recommended.
Littoral cell angioma (LCA) can range from no symptoms to a vague set of symptoms such as: abdominal pain, splenomegaly, thrombocytopenia, anemia, fever, chills, weakness and fatigue.
The main clinical finding is a splenic lesion.
The differential diagnosis of a splenic lesion is lymphoid, vascular, non lymphoid and tumor like which can be distinguished by pathology.
Ultrasound, computed tomography and magnetic resonance imaging are all acceptable modalities for imaging and diagnosing a splenic tumor; all of which are non-specific for LCA.
The splenic specimen is analyzed for abnormal littoral cells along with immunohistochemical stains to provide definitive diagnosis of LCA.
Treatment is surgical resection with close surveillance as a malignant variant is possible.
Over a hundred cases of LCA have been reported since 1991, research continues into the realm of pathological markers and surveillance is new territory with cases of malignant variants being reported.
Hemangioendothelioma is a term to describe a vascular neoplasm that may be considered benign as well as malignant.
This case teaches that there is malignant potential for LCA lesions of the spleen.
This is the well-written case report of LCA.
P- Reviewer: Kai K, Mueller WC, Sergi C S- Editor: Tian YL L- Editor: A E- Editor: Jiao XK
| 1. | Abbott RM, Levy AD, Aguilera NS, Gorospe L, Thompson WM. From the archives of the AFIP: primary vascular neoplasms of the spleen: radiologic-pathologic correlation. Radiographics. 2004;24:1137-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Fotiadis C, Georgopoulos I, Stoidis C, Patapis P. Primary tumors of the spleen. Int J Biomed Sci. 2009;5:85-91. [PubMed] |
| 3. | Kaza RK, Azar S, Al-Hawary MM, Francis IR. Primary and secondary neoplasms of the spleen. Cancer Imaging. 2010;10:173-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Rajabi P, Noorollahi H, Hani M, Bagheri M. Inflammatory pseudotumor of spleen. Adv Biomed Res. 2014;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Perry-Thornton E, Verly GP, Karkala J, Walker M. An unusual presentation of multiple myeloma: primary plasmacytoma of the spleen. J Natl Med Assoc. 1989;81:1095-1096, 1099. [PubMed] |
| 6. | Popp JA, Jones TC. Fibrosarcoma, Spleen. Berlin: Springer 1990; 216-219. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Lee JKT, Sagel S, Stanley RJ, Heiken JP. Computed Body Tomography with MRI Correlation. 4th ed. Lippincott William and Wilkins, 2006: 984-991. . |
| 8. | Palas J, Matos AP, Ramalho M. The spleen revisited: an overview on magnetic resonance imaging. Radiol Res Pract. 2013;2013:219297. [PubMed] |
| 9. | Mikami T, Saegusa M, Akino F, Machida D, Iwabuchi K, Hagiwara S, Okayasu I. A Kaposi-like variant of splenic angiosarcoma lacking association with human herpesvirus 8. Arch Pathol Lab Med. 2002;126:191-194. [PubMed] |
| 10. | Valls C, Cañas C, Turell LG, Pruna X. Hepatosplenic AIDS-related Kaposi’s sarcoma. Gastrointest Radiol. 1991;16:342-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Colović MD, Janković GM, Colović RB, Martinović-Cemerikić VM. Non-secretory solitary plasmacytoma of the spleen. Med Oncol. 1998;15:286-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Harris NL, Aisenberg AC, Meyer JE, Ellman L, Elman A. Diffuse large cell (histiocytic) lymphoma of the spleen. Clinical and pathologic characteristics of ten cases. Cancer. 1984;54:2460-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Bhatt S, Huang J, Dogra V. Littoral cell angioma of the spleen. AJR Am J Roentgenol. 2007;188:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Ben-Izhak O, Bejar J, Ben-Eliezer S, Vlodavsky E. Splenic littoral cell haemangioendothelioma: a new low-grade variant of malignant littoral cell tumour. Histopathology. 2001;39:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Meybehm M, Fischer HP. [Littoral cell angiosarcoma of the spleen. Morphologic, immunohistochemical findings and consideration of histogenesis of a rare splenic tumor]. Pathologe. 1997;18:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Bhatt S, Simon R, Dogra VS. Littoral cell angioma: sonographic and color Doppler features. J Ultrasound Med. 2007;26:539-542. [PubMed] |
| 17. | Harmon RL, Cerruto CA, Scheckner A. Littoral cell angioma: a case report and review. Curr Surg. 2006;63:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Ramdall RB, Alasio TM, Cai G, Yang GC. Primary vascular neoplasms unique to the spleen: littoral cell angioma and splenic hamartoma diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2007;35:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Rana N, Ming Z, Hui MS, Bin Y. Case Report: Littoral cell angioma of spleen. Indian J Radiol Imaging. 2009;19:210-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Tee M, Vos P, Zetler P, Wiseman SM. Incidental littoral cell angioma of the spleen. World J Surg Oncol. 2008;6:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Chourmouzi D, Psoma E, Drevelegas A. Littoral cell angioma, a rare cause of long standing anaemia: a case report. Cases J. 2009;2:9115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Johnson C, Goyal M, Kim B, Wasdahl D, Nazinitsky K. Littoral cell angioma. Clin Imaging. 2007;31:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Cosme A, Tejada A, Bujanda L, Vaquero M, Elorza JL, Ojeda E, Goikoetxea U. Littoral-cell angioma of the spleen: a case report. World J Gastroenterol. 2007;13:6603-6604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 12] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Qu ZB, Liu LX, Wu LF, Zhao S, Jiang HC. Multiple littoral cell angioma of the spleen: a case report and review of the literature. Onkologie. 2007;30:256-258. [PubMed] |
| 25. | Tatli S, Cizginer S, Wieczorek TJ, Ashley SW, Silverman SG. Solitary littoral cell angioma of the spleen: computed tomography and magnetic resonance imaging features. J Comput Assist Tomogr. 2008;32:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Ertan G, Tekes A, Mitchell S, Keefer J, Huisman TA. Pediatric littoral cell angioma of the spleen: multimodality imaging including diffusion-weighted imaging. Pediatr Radiol. 2009;39:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Suvajdzić N, Cemerikić-Martinović V, Saranović D, Petrović M, Popović M, Artiko V, Cupić M, Elezović I. Littoral-cell angioma as a rare cause of splenomegaly. Clin Lab Haematol. 2006;28:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Priego P, Rodríguez Velasco G, Griffith PS, Fresneda V. Littoral cell angioma of the spleen. Clin Transl Oncol. 2008;10:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Mac New HG, Fowler CL. Partial splenectomy for littoral cell angioma. J Pediatr Surg. 2008;43:2288-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Wang YJ, Li F, Cao F, Sun JB, Liu JF, Wang YH. Littoral cell angioma of the spleen. Asian J Surg. 2009;32:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Bi CF, Jiang LL, Li Z, Liu WP. [Littoral cell angioma of spleen: a clinicopathologic study of 17 cases]. Zhonghua Binglixue Zazhi. 2007;36:239-243. [PubMed] |
| 32. | Nagarajan P, Cai G, Padda MS, Selbst M, Kowalski D, Proctor DD, Chhieng D, Aslanian HR, Harigopal M. Littoral cell angioma of the spleen diagnosed by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol. 2011;39:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. 1991;15:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Levy AD, Abbott RM, Abbondanzo SL. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology. 2004;230:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Ziske C, Meybehm M, Sauerbruch T, Schmidt-Wolf IG. Littoral cell angioma as a rare cause of splenomegaly. Ann Hematol. 2001;80:45-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Goldfeld M, Cohen I, Loberant N, Mugrabi A, Katz I, Papura S, Noi I. Littoral cell angioma of the spleen: appearance on sonography and CT. J Clin Ultrasound. 2002;30:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Pilz JB, Sperschneider T, Lutz T, Loosli B, Maurer CA. Littoral cell angioma in main and accessory intrapancreatic spleen presenting as splenic rupture. Am J Surg. 2011;201:e15-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Yamane H, Ohashi K, Suwaki T, Takigawa N. Ruptured littoral cell angiosarcoma causing hemoperitoneum. Intern Med. 2012;51:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Bisceglia M, Sickel JZ, Giangaspero F, Gomes V, Amini M, Michal M. Littoral cell angioma of the spleen: an additional report of four cases with emphasis on the association with visceral organ cancers. Tumori. 1998;84:595-599. [PubMed] |
| 40. | Hanna T, Baumgarten D, Friedman T. Littoral Cell Angioma: a case and review of the literature. Radiology Case reports. 2011;6:324-326. |
| 41. | Arber DA, Strickler JG, Chen YY, Weiss LM. Splenic vascular tumors: a histologic, immunophenotypic, and virologic study. Am J Surg Pathol. 1997;21:827-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Ogembo JG, Milner DA, Mansfield KG, Rodig SJ, Murphy GF, Kutok JL, Pinkus GS, Fingeroth JD. SIRPα/CD172a and FHOD1 are unique markers of littoral cells, a recently evolved major cell population of red pulp of human spleen. J Immunol. 2012;188:4496-4505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | O’Malley DP, Kim YS, Weiss LM. Distinctive immunohistochemical staining in littoral cell angioma using ERG and WT-1. Ann Diagn Pathol. 2015;19:143-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Fernandez S, Cook GW, Arber DA. Metastasizing splenic littoral cell hemangioendothelioma. Am J Surg Pathol. 2006;30:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 45. | Kranzfelder M, Bauer M, Richter T, Rudelius M, Huth M, Wagner P, Friess H, Stadler J. Littoral cell angioma and angiosarcoma of the spleen: report of two cases in siblings and review of the literature. J Gastrointest Surg. 2012;16:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Rosso R, Paulli M, Gianelli U, Boveri E, Stella G, Magrini U. Littoral cell angiosarcoma of the spleen. Case report with immunohistochemical and ultrastructural analysis. Am J Surg Pathol. 1995;19:1203-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |