Published online Sep 16, 2014. doi: 10.12998/wjcc.v2.i9.474
Revised: June 24, 2014
Accepted: July 15, 2014
Published online: September 16, 2014
Processing time: 256 Days and 3.1 Hours
The champagne bottle neck (CBN) sign refers to a reduction in the diameter of the proximal portion of the internal carotid artery that resembles a CBN, and is a characteristic feature of Moyamoya disease. A 43-year-old woman with an infarction of the posterior limb of the left internal capsule was diagnosed with Moyamoya syndrome associated with Graves’ disease. The CBN sign was observed bilaterally. Cerebral revascularization surgery was performed, including left-sided superficial temporal artery to middle cerebral artery anastomosis. During four years of follow-up, she maintained a euthyroid state and did not have any further cerebral ischemic events. The CBN signs remained unchanged on both sides during this time. This is the first report of the CBN sign in a patient with Moyamoya syndrome associated with Graves’ disease.
Core tip: The champagne bottle neck (CBN) sign reflects a reduction in the diameter of the proximal portion of the internal carotid artery that resembles a CBN, and is a characteristic feature of Moyamoya disease. This case describes the first report of bilateral CBN signs in a 43-year-old woman diagnosed with Moyamoya syndrome associated with Graves’ disease. Cerebral revascularization surgery was performed on the patient, and the CBN signs remained unchanged throughout four years of follow-up.
- Citation: Shimogawa T, Morioka T, Sayama T, Hamamura T, Yasuda C, Arakawa S. Champagne bottle neck sign in a patient with Moyamoya syndrome. World J Clin Cases 2014; 2(9): 474-477
- URL: https://www.wjgnet.com/2307-8960/full/v2/i9/474.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i9.474
Moyamoya disease (MMD) is a cerebrovascular disorder characterized by progressive bilateral stenosis or occlusion of the distal portion of the internal carotid artery (ICA) and proximal portions of the middle cerebral artery (MCA) and anterior cerebral artery (ACA), together with the formation of net-like collateral vessels in the basal ganglia[1,2]. The rarely observed vasculopathy that is characteristic of this disease is seen associated with pathologic conditions such as arteriosclerosis, Down’s syndrome, von Recklinghausen’s disease, and X-ray irradiation in Moyamoya syndrome (MMS)[3]. Several cases of Moyamoya vasculopathy associated with Graves’ disease have been reported[4-7]. Graves’ disease is a female-dominant autoimmune thyroid disease characterized by the formation of autoantibodies to thyroid-stimulating hormone (TSH) receptors, resulting in continuous stimulation of the thyroid gland and hyperthyroidism[4].
In 2006, Yasaka et al[8] reported that ultrasonography in patients with MMD showed a rapid reduction in the diameter of the proximal ICA to less than half of the diameter of the common carotid artery, giving the appearance of a champagne bottle neck (CBN). The CBN sign occurred in 74% of patients with MMD, but has not been reported in patients with MMS. We report a case involving a patient with MMS associated with Graves’ disease, in whom the CBN sign was observed. The results of long-term follow-up after cerebral revascularization surgery are also presented.
A 43-year-old Japanese woman presented with weakness of the right extremities, which she first noticed when she awoke in the morning. She had a six-month history of fatigue and weight loss. On admission, her blood pressure was 117/72 mmHg and her heart rate was 125 beats/min. Physical examination revealed a right-sided carotid bruit. No goiter or exophthalmos was observed. A neurologic examination showed mild right hemiparesis and dysarthria.
Laboratory data demonstrated hyperthyroidism with a TSH level of 0.015 μU/mL (normal range: 0.35-4.94 μU/mL), free T3 level of 26.42 pg/mL (normal range: 1.71-3.71 pg/mL), and free T4 level of 4.37 ng/dL (normal range: 0.70-1.48 ng/dL). Her TSH receptor antibody level was 36.7% (normal: < 15%). Ultrasonography of the thyroid gland showed a diffuse goiter, and thyroid scintigraphy was positive. She was diagnosed with hyperthyroidism due to Graves’ disease, and antithyroid drug treatment with methimazole and potassium iodide was started.
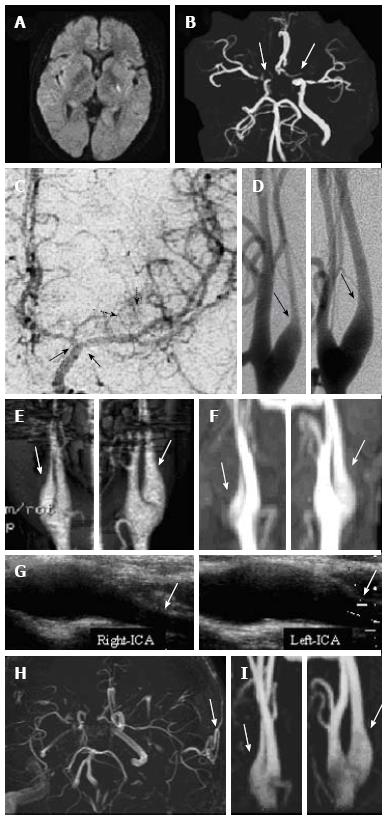
Diffusion-weighted magnetic resonance imaging on admission showed a hyperintense area at the left posterior limb of the internal capsule (Figure 1A). Magnetic resonance angiography (MRA) and three-dimensional computed tomography angiography showed bilateral severe stenosis of the distal ICA and proximal MCA and ACA (Figure 1B). Digital subtraction angiography showed Moyamoya vessels in the left basal ganglia and bilateral stenosis of the distal ICA and proximal MCA and ACA (Figure 1C). No Moyamoya vessels were observed on the right side. Bilateral narrowing of the proximal ICA consistent with the CBN sign was also observed on digital subtraction angiography (Figure 1D), three-dimensional computed tomography angiography (Figure 1E), and ultrasonography (Figure 1F).
The patient was diagnosed with an acute infarction of the posterior limb of the internal capsule due to Moyamoya syndrome (Suzuki stage 3 on the right side and stage 1 on the left side[4]) associated with Graves’s disease. She was treated with ozagrel, edaravone and heparin, and subsequently with aspirin and warfarin. Antithyroid drug treatment with methimazole and potassium iodide was continued.
The patient’s right hemiparesis and dysarthria were resolved two months later. She had experienced no additional cerebral ischemic events and was euthyroid with a TSH level of < 0.016 μU/mL and a free T3 level of 3.02 pg/mL. MRA showed no change in the bilateral CBN signs (Figure 1G) or the bilateral severe stenosis of the distal ICA and proximal MCA and ACA. Single-photon emission computed tomography with N-isopropyl-p-[123I]-iodoamphetamine (IMP-SPECT) showed a markedly compromised vascular reserve in the left MCA territory and slightly decreased cerebral blood flow (41.69 and 43.80 mL/100 g/min in the left and right MCA territories, respectively). The regional cerebrovascular reactivity after acetazolamide loading was 7.10% in the left MCA territory and 11.79% in the right MCA territory.
To treat the perfusion insufficiency in the left MCA territory, left superficial temporal artery to MCA anastomosis, encephalo-duro-arterio-synangiosis and encephalo-myo-synangiosis were performed. The antiplatelet and anticoagulation medications were discontinued postoperatively.
MRA at one month after the cerebral revascularization surgery showed a patent bypass (Figure 1H). There was no change in the bilateral stenosis of the distal ICA and proximal MCA and ACA. IMP-SPECT showed that cerebral blood flow was improved in the left MCA territory (45.75 and 44.41 mL/100 g/min in the left and right MCA territories, respectively) and that regional cerebrovascular reactivity was improved in the left MCA territory (13.07% and 17.45% in the left and right MCA territories, respectively).
During four years of follow-up after surgery, the patient’s euthyroid state was maintained with antithyroid therapy and she experienced no additional cerebral ischemic events. An MRA at four years after surgery showed no change in the bilateral CBN signs (Figure 1I).
Although the CBN sign is a characteristic feature of MMD[8], no previous reports have described the clinical significance of this sign or the specific underlying pathophysiologic mechanisms. Histopathologic examination shows eccentrically laminated thickening of the intima of the major intracranial arteries in patients with MMD[9]. Fibrocellular intimal thickening is also observed in other arteries such as the extracranial ICAs, external carotid arteries, pulmonary arteries, renal arteries and coronary arteries[10-12]. At the carotid bifurcation, there is a transitional zone between the elastic portion of the carotid arteries (located > 5 mm proximal to the bifurcation) and the muscular portion of the carotid arteries (located > 15 mm distal to the bifurcation)[13,14]. It is speculated that the muscular portion is more commonly affected than the elastic portion because of its thinner intimal membrane[13], and that the stenosis extends from the distal to the proximal ICA. The narrowing at the transitional zone results in the CBN sign.
This is the first report of the CBN sign in a patient with MMS. Although the association between MMS and Graves’ disease is not well understood[3-7], it has been hypothesized that the increased vascular reactivity resulting from hyperthyroidism causes damage to the arterial wall, leading to MMD[15-17]. The CBN sign may reflect intimal thickening of the extracranial ICA.
In the series of patients with MMD reported by Yasaka et al[8], the relationships among the stage of MMD, the presence of the CBN sign, and chronologic changes in the CBN sign, were not reported. In our patient, the stage of MMS was more advanced on the left (the symptomatic side) than on the right. Moyamoya vessels were not observed on the right side, and perfusion reserve was impaired in the left MCA territory. However, the CBN sign was observed equally on both sides, and did not change between the hyperthyroid and euthyroid states. During the four years after cerebral revascularization surgery, no changes were observed in the CBN signs. It has previously been reported that Moyamoya-like vascular changes and stenosis of the intracranial arteries and do not occur in patients with MMS associated with Graves’ disease after antithyroid treatment and cerebral revascularization[3-5,18]. Likewise, the changes causing the CBN sign are irreversible.
A 43-year-old Japanese woman presented with weakness of the right extremities and had a six-month history of fatigue and weight loss.
The patient’s heart rate was 125 beats/min and a neurologic examination showed mild right hemiparesis and dysarthria.
Brain infarction; Intracerebral hemorrhage; Brain tumor.
Laboratory data demonstrated hyperthyroidism with a thyroid-stimulating hormone level of 0.015 μU/mL, free T3 level of 26.42 pg/mL, free T4 level of 4.37 ng/dL, and a thyroid-stimulating hormone receptor antibody level of 36.7%.
Diffusion-weighted magnetic resonance imaging on admission showed a hyperintense area at the left posterior limb of the internal capsule, and digital subtraction angiography showed Moyamoya vessels in the left basal ganglia and bilateral stenosis of the distal internal carotid artery and proximal middle and anterior cerebral arteries. Bilateral narrowing of the proximal internal carotid artery consistent with the champagne bottle neck (CBN) sign was also observed on three-dimensional computed tomography angiography and ultrasonography.
The patient was diagnosed with acute infarction of the posterior limb of the internal capsule due to Moyamoya syndrome associated with Graves’s disease.
Revascularization was performed following antithyroid drug treatment.
Ultrasonography in patients with Moyamoya disease showed a rapid reduction in the diameter of the proximal internal carotid artery to less than half of the diameter of the common carotid artery (giving the appearance of a CBN), although the relationships among the disease stage, presence of the CBN sign, and chronologic changes in the CBN sign were not reported.
The CBN signs were not affected by the change between the hyperthyroid and euthyroid states. Furthermore, no changes in the CBN signs were observed after cerebral revascularization surgery, demonstrating their irreversibility.
This is the first report of the CBN sign in a patient with Moyamoya syndrome associated with Graves’ disease.
P- Reviewer: Starke R S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Kuroda S, Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol. 2008;7:1056-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 2. | Burke GM, Burke AM, Sherma AK, Hurley MC, Batjer HH, Bendok BR. Moyamoya disease: a summary. Neurosurg Focus. 2009;26:E11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Endo H, Fujimura M, Niizuma K, Shimizu H, Tominaga T. Efficacy of revascularization surgery for moyamoya syndrome associated with Graves’ disease. Neurol Med Chir (Tokyo). 2010;50:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Im SH, Oh CW, Kwon OK, Kim JE, Han DH. Moyamoya disease associated with Graves disease: special considerations regarding clinical significance and management. J Neurosurg. 2005;102:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Tokimura H, Tajitsu K, Takashima H, Hirayama T, Tsuchiya M, Takayama K, Arita K. Familial moyamoya disease associated with Graves’ disease in a mother and daughter. Two case reports. Neurol Med Chir (Tokyo). 2010;50:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Malik S, Russman AN, Katramados AM, Silver B, Mitsias PD. Moyamoya syndrome associated with Graves’ disease: a case report and review of the literature. J Stroke Cerebrovasc Dis. 2011;20:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Li D, Yang W, Xian P, Liu P, Bao X, Zong R, Duan L. Coexistence of moyamoya and Graves’ diseases: the clinical characteristics and treatment effects of 21 Chinese patients. Clin Neurol Neurosurg. 2013;115:1647-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Yasaka M, Ogata T, Yasumori K, Inoue T, Okada Y. Bottle neck sign of the proximal portion of the internal carotid artery in moyamoya disease. J Ultrasound Med. 2006;25:1547-152; quiz 1547-152;. [PubMed] |
| 9. | Yamashita M, Oka K, Tanaka K. Histopathology of the brain vascular network in moyamoya disease. Stroke. 1983;14:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 179] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Hoshimaru M, Kikuchi H. Involvement of the external carotid arteries in moyamoya disease: neuroradiological evaluation of 66 patients. Neurosurgery. 1992;31:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Ou P, Dupont P, Bonnet D. Fibromuscular dysplasia as the substrate for systemic and pulmonary hypertension in the setting of Moya-Moya disease. Cardiol Young. 2006;16:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Yang SH, Li B, Wang CC, Zhao JZ. Angiographic study of moyamoya disease and histological study in the external carotid artery system. Clin Neurol Neurosurg. 1997;99 Suppl 2:S61-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hori E, Hayashi N, Hamada H, Masuoka T, Kuwayama N, Hirashima Y, Origasa H, Ohtani O, Endo S. A development of atheromatous plaque is restricted by characteristic arterial wall structure at the carotid bifurcation. Surg Neurol. 2008;69:586-590; discussion 590-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Janzen J, Lanzer P, Rothenberger-Janzen K, Vuong PN. Variable extension of the transitional zone in the medial structure of carotid artery tripod. Vasa. 2001;30:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Honda H, Iwata T, Mochizuki T, Kogo H. Changes in vascular reactivity induced by acute hyperthyroidism in isolated rat aortae. Gen Pharmacol. 2000;34:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Inaba M, Henmi Y, Kumeda Y, Ueda M, Nagata M, Emoto M, Ishikawa T, Ishimura E, Nishizawa Y. Increased stiffness in common carotid artery in hyperthyroid Graves’ disease patients. Biomed Pharmacother. 2002;56:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Squizzato A, Gerdes VE, Brandjes DP, Büller HR, Stam J. Thyroid diseases and cerebrovascular disease. Stroke. 2005;36:2302-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Utku U, Asil T, Celik Y, Tucer D. Reversible MR angiographic findings in a patient with autoimmune Graves disease. AJNR Am J Neuroradiol. 2004;25:1541-1543. [PubMed] |