Published online May 16, 2014. doi: 10.12998/wjcc.v2.i5.160
Revised: February 13, 2014
Accepted: April 11, 2014
Published online: May 16, 2014
Processing time: 174 Days and 21.9 Hours
The discovery of a strong association between hepatitis C virus (HCV) infection and mixed cryoglobulinemia (MC) has led to an increasingly rare diagnosis of idiopathic essential MC (EMC). The incidence of EMC is high in regions where there is a comparatively low HCV infection burden and low in areas of high infection prevalence, including HCV. The diagnosis of EMC requires an extensive laboratory investigation to exclude all possible causes of cryoglobulin formation. In addition, although cryoglobulin testing is simple, improper testing conditions will result in false negative results. Here, we present a 46-year-old female patient with a case of EMC with dermatological and renal manifestations, highlighting the importance of extensive investigation to reach a proper diagnosis. We review the need for appropriate laboratory testing, which is often neglected in clinical practice and which can result in false negative results. This review also emphasizes the significance of an extended testing repertoire necessary for better patient management. Despite a strong association of MC with HCV infection and other causes that lead to cryoglobulin formation, EMC remains a separate entity. Correct diagnosis requires proper temperature regulation during sample handling, as well as characterization and quantification of the cryoprecipitate. Inclusion of rheumatoid factor activity and complement levels in the cryoglobulin test-panel promotes better patient management and monitoring. Consensus guidelines should be developed and implemented for cryoglobulin detection and the diagnosis of cryoglobulinemic syndrome, which will reduce variability in inter-laboratory reporting.
Core tip: The diagnosis of essential mixed cryoglobulinemia (EMC) requires thorough laboratory investigation to exclude all possible causes of cryoglobulin formation. Although cryoglobulin testing is simple, it requires careful temperature regulation to avoid false negative results. The testing panel should also include cryoglobulin quantification and characterization, rheumatoid factor activity and complement levels, to better facilitate patient management. Furthermore, there is a need to develop and implement consensus guidelines for laboratory and clinical diagnoses of EMC.
- Citation: Anis S, Abbas K, Mubarak M, Ahmed E, Bhatti S, Muzaffar R. Vasculitis with renal involvement in essential mixed cryoglobulinemia: Case report and mini-review. World J Clin Cases 2014; 2(5): 160-166
- URL: https://www.wjgnet.com/2307-8960/full/v2/i5/160.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i5.160
Cryoglobulins (CGs) are abnormal proteins/immunoglobulins (Igs) that precipitate out of serum at temperatures below 37 °C[1]. Initially identified by Winthrobe and Buell in 1933 and later named by Lerner et al[2], CGs are found in many disorders ranging from autoimmune and infectious diseases to malignancies[3-5]. Cryoglobulinemia, the presence of CGs in blood, is significant only when the associated symptoms are present[6,7]. Mixed cryoglobulinemia (MC) is strongly associated with hepatitis C virus (HCV) infection[8-11]. Other causes, including autoimmune disorders and other infections, can lead to MC[12]. When the cause for MC cannot be identified, the disease is termed as idiopathic or essential mixed cryoglobulinemia (EMC)[13-15]. An extensive laboratory investigation is required to rule out the known conditions associated with cryoglobulinemic vasculitis to impart a proper diagnosis of EMC[3,4,16]. Here, we present a 46-year-old female patient with EMC and briefly review the current laboratory methods for diagnosing this syndrome.
A 46-year-old female patient presented to the Department of Nephrology after suffering from anuria and renal failure for ten days. Prior to admission at our institute, the patient had already undergone four sessions of dialysis in another hospital. The only significant medical history for the patient was a laparoscopic cholecystectomy she received two months prior. The patient had no history of hypertension, diabetes mellitus or other renal disease.
Upon routine examination, the patient was pale, icteric, apyrexial and normotensive, with facial and pedal edema. Palpable purpura was present on both hands. No other abnormalities were revealed during the remainder of the systemic examination. Laboratory investigations revealed normochromic and normocytic anemia, elevated total leukocyte counts with neutrophilia, and thrombocytopenia. Renal and liver functions were abnormal and proteinuria was present (+2 on a dipstick) (Table 1). A chest X-ray and an abdominal ultrasound were normal. There was no evidence of malignancy or infections upon bone marrow biopsy, and all requested cultures (blood, urine, and bone marrow) to rule out infections were negative. A provisional diagnosis of vasculitis was made.
| Laboratory investigation | Test results (reference ranges) |
| Complete blood cell counts | |
| Hemoglobin | 8.5 g/dL (11.5-15.4 g/dL) |
| White blood cell count | 15200 × 109/L (4.0-11.0 × 109/L) |
| Neutrophils | 87% (40%-80%) |
| Lymphocytes | 10% (20%-40%) |
| Platelets | 23000/L (150-400 × 109/L) |
| Coagulation profile | |
| APTT | 28 s (25.8 s) |
| INR | 1.98 (< 1.35) |
| Renal functions | |
| Urea | 88 mg/dL (15-39 mg/dL) |
| Creatinine | 6.2 mg/dL (0.5-1.5 mg/dL) |
| Liver functions | |
| Total bilirubin | 6.1 mg/dL (0.2-1.0 mg/dL) |
| Direct bilirubin | 3.2 mg/dL (0-0.25 mg/dL) |
| Alkaline phosphatase | 202 U/mL (32-92 U/mL) |
| Alanine amino transferase | 10 U/mL (10-40 U/mL) |
| Gamma glutamyl transferase | 204 U/mL (7-64 U/mL) |
| Bone chemistry | |
| Calcium | 7.5 mg/dL (8.4-10.2 mg/dL) |
| Phosphorus | 4.4 mg/dL (2.5-4.0 mg/dL) |
| Albumin | 2.2 mg/dL (3.5-5.0 mg/dL) |
| Urine detailed report | Anuric on admission, later showed +2 proteinuria; no red blood cells, no cast |
| Immunological work-up | |
| Complement 3 | 0.4 g/L (0.79-1.52 g/L) |
| Complement 4 | 0.01 g/L (0.16-0.38 g/L) |
| Anti-nuclear antibodies | Negative |
| ANCA | Negative |
| Rheumatoid factor activity | Positive: 859 IU/mL (cut off < 20 IU/mL) |
| Anti-cyclic citrullinated peptide | Negative |
| Anti-extractable nuclear antigens | Weakly positive for anti-Ro52 |
| C-reactive protein | 0.1 mg/dL (cut-off < 0.7 mg/dL) |
| Viral markers | |
| HBsAg | Negative |
| Anti-HCV | Negative |
| Anti-HIV | Negative |
| HCV RNA | Not detected |
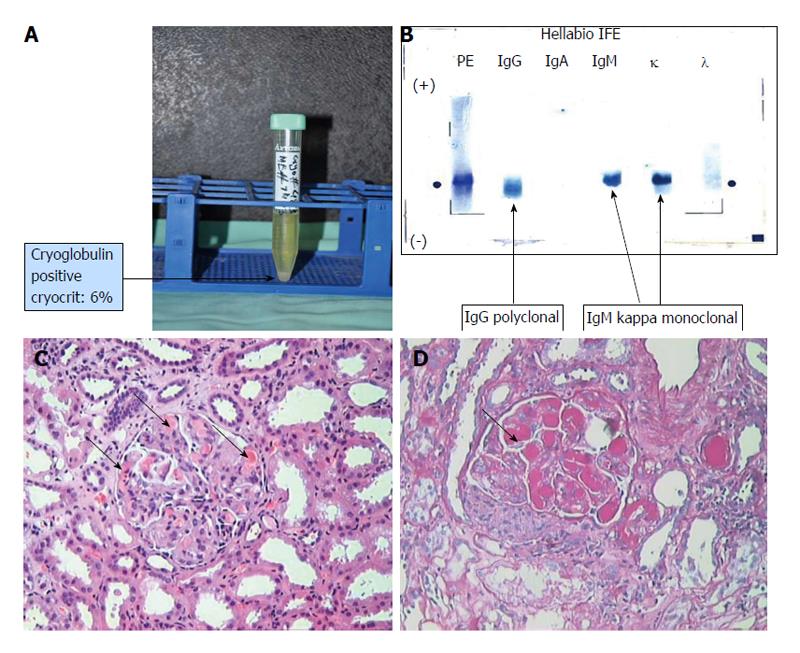
In the absence of infections and malignancies, further examinations were carried out (Table 1). An immunological work-up showed low complement levels (C3 and C4) and a negative anti-nuclear antibody test, which remained negative at a 6-mo follow-up. Type II CGs were detected, with a cryocrit of 6% (Figure 1A and B). Tests for anti-HCV antibodies and HCV RNA were negative. Other tests to evaluate vasculitis were also negative (Table 1). Renal biopsy showed evidence of cryoglobulinemic glomerulonephritis (Figure 1C and D).
Before vasculitis was suspected, the patient was empirically managed with antibiotics and hemodialysis, but did not show improvement. After the diagnosis of cryoglobulinemic glomerulonephritis was made, the patient was treated with intravenous solumedrol (15 mg/kg body weight) for three days, monthly intravenous injections of cyclophosphamide (15 mg/kg body weight) for five months, and plasmapheresis. After 14 plasmapheresis sessions, the patient’s renal functions normalized (serum creatinine 1.2 mg/dL) and the cryocrit decreased to 1%. Cryocrit levels subsequently decreased to less than 1% over a period of six months. Normal renal function was found to be maintained at the one-year follow-up examination.
Brouet et al[17] immunochemically classified CGs into three types. Type I CGs are characterized by the presence of only a monoclonal component, mostly IgMκ, and are usually found in patients with lymphoid malignancies such as multiple myeloma. Mixed CGs are comprised of type II, characterized by the presence of polyclonal Igs with a monoclonal component, and type III, which contain only polyclonal Igs[17]. Mixed CGs are also associated with rheumatoid factor (RF) activity[7,18,19].
Before the discovery of HCV infection, most cases of MC were defined as EMC, without an underlying identifiable cause[20]. In the early 1990s, HCV became recognized as the major cause of CG formation, with approximately 90% of MC patients having the virus[3]. It has been reported that approximately 50% of HCV-infected patients develop CGs in their blood[6]. The viral particles have also been detected in cryoprecipitates[21,22]. Since this discovery, the diagnosis of EMC has become rare[10,16,23].
Autoimmune or connective tissue diseases[13,14,24,25], lymphoproliferative disorders[26] and other infections such as bacterial, viral and parasitic[27] infections, have been attributed to MC development. However, 5%-48% of cases with cryoglobulinemic syndrome have still been reported as EMC[28-30]. The incidence of EMC is higher in regions with a low prevalence of HCV infection, and lower in areas with high HCV prevalence[4]. In Pakistan, more than ten million people are infected with HCV[31,32] and there is a high burden of other infections, especially in immunocompromised populations[33], which makes the risk for EMC lower. Therefore, an extensive and thorough examination is required to rule out other causes of cryoglobulinemia and properly assign an EMC diagnosis[34,35].
Cryoglobulinemic syndrome was first described by Meltzer et al[18] in 1966 as a triad of symptoms including purpura, arthralgia and weakness. The syndrome may also involve the visceral organs, including hepatosplenomegaly[36], glomerulonephritis[37] and lymphadenopathy[30]. The presence of CGs is not always associated with clinical features[24], and low levels of CGs have been found in HCV-infected patients without associated symptoms[4,6,7]. During renal replacement therapy for end stage renal disease, low levels of CGs have been detected in a substantial number of non-infected and HCV-infected patients, with and without symptoms[33].
The incidence and presentation of renal manifestations in HCV-associated cryoglobulinemia is well documented[10,11]. In non-HCV cryoglobulinemia, including EMC, the evidence of renal manifestations is predominantly confined to case reports[29,38-42]. It is important to note that most of the data on renal manifestations in EMC have come from studies on French populations[16,28,30,34]. In a multicenter French study comprised of 20 HCV-negative patients with renal manifestations, 50% of the patients had EMC[16]. In this study, microscopic hematuria and hypertension were the most common presenting clinical features, followed by nephrotic range proteinuria and renal insufficiency. In a separate French study, which contained 33 patients with type II CGs, 13 (39%) of the patients had EMC. Of these patients, eight (62%) had renal involvement, with four of those suffering from renal insufficiency[34]. In a Dutch study of 22 non-HCV patients with type II CGs, seven (32%) were found to have EMC, of which six patients had renal involvement[35].
Detection of CGs requires stringent temperature control, a fact that cannot be stressed enough. It is imperative to maintain the sample temperature at 37 °C from the time the sample is collected until separation of the serum[43]. Failure to keep samples at 37 °C will result in false negative results[4,44,45]. In our own experience, centrifuges even those lacking a heater-with a wide temperature range (e.g., 0 °C-40 °C) can be used to separate serum at 37 °C. This can be achieved by preheating the instrument with an initial dry run at > 37 °C and then immediately transferring the samples from a 37 °C incubator to the centrifuge. Once centrifugation is complete, the samples should be immediately taken out of the centrifuge and placed in a 37 °C incubator until the supernatant can be separated. Adherence to this protocol helps to avoid red blood cell contamination, which can hinder the visibility of CGs.
Although cryoglobulinemic syndrome was first identified in 1933, the significance of a proper CG detection assay and awareness for the condition is still not fully recognized. A large European survey of 140 laboratories revealed that only 37 (26%) were following the appropriate standard procedure for CG detection[45]. The reporting of CG positive results should include quantification and characterization of these proteins, RF activity and complement (C3 and C4) levels[5,44,45]. Although CG concentration does not correlate well with disease activity, CG levels, along with RF activity and complement levels, are useful in monitoring the disease[5,19].
This case report highlights the importance for proper and thorough investigation of a patient in order to diagnose cryoglobulinemic syndrome in the absence of infections (including HCV) and other known causes of MC. Furthermore, the quantification and characterization of CGs is important, as the decrease in cryocrit level in the present case correlated well with clinical remission. Although repeated anti-nuclear antibody tests were negative, there was a weak positivity for anti-Ro52 antibodies, which can be found in various conditions, including cryoglobulinemia[46]. Similarly, a low C-reactive protein has also been observed in the serum of patients with MC, not related to systemic lupus erythematosus[19,47].
CG quantification can be done in various ways, including total protein or immunoglobulin content of cryoprecipitates, cryocrit determination[45], gel-based semi-quantitative tests[48] and other methods[49,50]. Determination of levels using a cryocrit is less cumbersome and more informative, though it should be noted that a cryocrit is less sensitive and specific than quantification of washed cryoproteins[44]. CG characterization provides valuable information that aids in long-term patient management[45,49]. Patients with type II CGs should be closely monitored for B cell lymphoma development, especially in patients that are difficult to treat[6,15]. Lack of standardization and an incomplete testing repertoire inadvertently lead to distrust of laboratory results by the treating physicians and undermine the significance of CG detection in suspected cases, which may poorly affect patient management[4,45,49,50]. This is very important given that better treatment options are increasingly becoming available to manage this rare yet troublesome disorder[41,51,52].
Varied clinical presentation and insignificant correlation of CG concentration with clinical manifestations have made the diagnosis of cryoglobulinemic syndrome quite difficult. Thus, various diagnostic and classification criteria have been proposed to facilitate in the diagnosis of this condition[7,53,54]. Diagnostic criteria are based on major and minor laboratory and clinical features[54], and preliminary classification criteria have been proposed, including a questionnaire regarding symptoms, laboratory findings (including CG types, complement levels, RF activity and HCV presence) and organ involvement[7]. Diagnostic and classification criteria indicate that the presence of CGs in serum is the gold standard for a cryoglobulinemic vasculitis diagnosis. Moreover, the presence of clinical features consistent with this syndrome in the absence of CGs warrants repeat testing for these proteins given the possibility for false negative results[7,53].
In conclusion, despite a strong association of MC with HCV infection and other causes that can lead to CG formation, EMC remains a separate entity. Accurate diagnosis requires a thorough laboratory investigation to rule out the known causes of MC. Correct laboratory diagnosis not only requires proper handling of the samples for CG detection, but also quantification of the amount of cryoprecipitate and identification of the type of CGs present. Other tests, such as RF activity and complement levels (C3 and C4), should be included in the testing panel to ensure better patient management and monitoring. There is a need to develop and implement consensus guidelines for the detection of CGs and cryoglobulinemic syndrome to reduce variability in inter-laboratory reporting and to establish the diagnostic criteria for this clinical syndrome.
A 46-year-old female presented with anuria, acute renal failure and palpable purpura.
The patient was diagnosed with essential mixed cryoglobulinemic syndrome.
Vasculitis causing acute renal failure was ruled out.
The following laboratory tests were acquired from the patient: negative cultures, anti-nuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-hepatitis C virus (HCV) and HCV RNA, and positive cryoglobulins (CGs) with low C3 and C4 levels and high rheumatoid factor (RF) activity.
The patient had normal chest X-ray and abdominal ultrasound reports.
Hematoxylin and eosin staining, in addition to periodic acid-Schiff stains from a renal biopsy, showed evidence of cryoglobulinemic glomerulonephritis.
The patient was treated with intravenous injections of solumedrol and cyclophosphamide, and plasmapheresis, which improved her condition.
Essential mixed cryoglobulinemia (EMC) is rare and requires a thorough examination to rule out other known causes. A complete work-up for cryoglobulinemic syndrome, including quantification and characterization of cryoprecipitates, complement levels and RF activity, is required for proper management and monitoring.
Cryoglobulinemia is a disorder in which CGs precipitate out of the blood at temperatures lower than 37 °C and is often associated with hepatitis C viral infection.
Early recognition, extensive diagnostic work-up and proper patient management, including regular follow-ups and immunological monitoring, resulted in a favorable patient outcome in this study.
This case report with a mini-review highlights the importance of a thorough investigation for the rare and often neglected diagnosis of EMC. This article will be of great benefit to clinicians as it increases the awareness regarding the clinical utility and proper testing and interpretation of cryoglobulin detection assays.
P- Reviewers: Boffano P, Cho SY S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Morra E. Cryoglobulinemia. Hematology Am Soc Hematol Educ Program. 2005;368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Lerner AB, Watson CJ. Studies of cryoglobulins; unusual purpura associated with the presence of a high concentration of cryoglobulin (cold precipitable serum globulin). Am J Med Sci. 1947;214:410-415. [PubMed] |
| 3. | Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012;379:348-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Damoiseaux J, Cohen Tervaert JW. Diagnostics and Treatment of Cryoglobulinaemia: It Takes Two to Tango. Clin Rev Allergy Immunol. 2013;Epub ahead of print. [PubMed] |
| 5. | Shihabi ZK. Cryoglobulins: an important but neglected clinical test. Ann Clin Lab Sci. 2006;36:395-408. [PubMed] |
| 6. | Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | De Vita S, Soldano F, Isola M, Monti G, Gabrielli A, Tzioufas A, Ferri C, Ferraccioli GF, Quartuccio L, Corazza L. Preliminary classification criteria for the cryoglobulinaemic vasculitis. Ann Rheum Dis. 2011;70:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Lauletta G, Russi S, Conteduca V, Sansonno L. Hepatitis C virus infection and mixed cryoglobulinemia. Clin Dev Immunol. 2012;2012:502156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Viganò M, Lampertico P, Rumi MG, Folli C, Maggioni L, Morabito A, Del Ninno E, Cicardi M, Colombo M. Natural history and clinical impact of cryoglobulins in chronic hepatitis C: 10-year prospective study of 343 patients. Gastroenterology. 2007;133:835-842. [PubMed] |
| 10. | Fabrizi F. Hepatitis C virus, cryoglobulinemia, and kidney: novel evidence. Scientifica (Cairo). 2012;2012:128382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Campise M, Tarantino A. Glomerulonephritis in mixed cryoglobulinaemia: what treatment? Nephrol Dial Transplant. 1999;14:281-283. [PubMed] |
| 12. | Bryce AH, Kyle RA, Dispenzieri A, Gertz MA. Natural history and therapy of 66 patients with mixed cryoglobulinemia. Am J Hematol. 2006;81:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | García-Carrasco M, Ramos-Casals M, Cervera R, Trejo O, Yagüe J, Sisó A, Jiménez S, de La Red G, Font J, Ingelmo M. Cryoglobulinemia in systemic lupus erythematosus: prevalence and clinical characteristics in a series of 122 patients. Semin Arthritis Rheum. 2001;30:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Ostojic P. Cryoglobulinemic vasculitis in systemic sclerosis successfully treated with mycophenolate mofetil. Rheumatol Int. 2014;34:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | De Vita S, Quartuccio L, Salvin S, Corazza L, Zabotti A, Fabris M. Cryoglobulinaemia related to Sjogren’s syndrome or HCV infection: differences based on the pattern of bone marrow involvement, lymphoma evolution and laboratory tests after parotidectomy. Rheumatology (Oxford). 2012;51:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Matignon M, Cacoub P, Colombat M, Saadoun D, Brocheriou I, Mougenot B, Roudot-Thoraval F, Vanhille P, Moranne O, Hachulla E. Clinical and morphologic spectrum of renal involvement in patients with mixed cryoglobulinemia without evidence of hepatitis C virus infection. Medicine (Baltimore). 2009;88:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775-788. [PubMed] |
| 18. | Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinemia--a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40:837-856. [PubMed] |
| 19. | Gorevic PD. Rheumatoid factor, complement, and mixed cryoglobulinemia. Clin Dev Immunol. 2012;2012:439018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Dammacco F, Sansonno D, Piccoli C, Tucci FA, Racanelli V. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Barone M, Licinio R, Amoruso A, Viggiani MT, Larocca AM, Di Leo A. Lesson from an intriguing case of cryoglobulinemia. World J Gastroenterol. 2013;19:304-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 22. | Della Rossa A, Baldini C, Tavoni A, Bombardieri S. How HCV has changed the approach to mixed cryoglobulinemia. Clin Exp Rheumatol. 2009;27:S115-S123. [PubMed] |
| 23. | Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F, Sansonno D. Impact of Cryoglobulinemic Syndrome on the Outcome of Chronic Hepatitis C Virus Infection: A 15-Year Prospective Study. Medicine (Baltimore). 2013;Epub ahead of print. [PubMed] |
| 24. | Monti G, Saccardo F, Castelnovo L, Novati P, Sollima S, Riva A, Sarzi-Puttini P, Quartuccio L, De Vita S, Galli M. Prevalence of mixed cryoglobulinaemia syndrome and circulating cryoglobulins in a population-based survey: the Origgio study. Autoimmun Rev. 2014;13:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Zivanovic D, Dobrosavljevic D, Nikolic M, Bonaci-Nikolic B. Cryoglobulins and multispecific antineutrophil cytoplasmic antibodies in propylthiouracil-induced necrotizing cutaneous vasculitis--a new association. Eur J Dermatol. 2012;22:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 302] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Pagnoux C, Cohen P, Guillevin L. Vasculitides secondary to infections. Clin Exp Rheumatol. 2006;24:S71-S81. [PubMed] |
| 28. | Rieu V, Cohen P, André MH, Mouthon L, Godmer P, Jarrousse B, Lhote F, Ferrière F, Dény P, Buchet P. Characteristics and outcome of 49 patients with symptomatic cryoglobulinaemia. Rheumatology (Oxford). 2002;41:290-300. [PubMed] |
| 29. | Rozin AP, Lewin M, Braun-Moscovici Y, Itzhak OB, Bergman R, Balbir-Gurman A. Essential mixed cryoglobulinemia type II. Clin Exp Rheumatol. 2006;24:329-332. [PubMed] |
| 30. | Terrier B, Krastinova E, Marie I, Launay D, Lacraz A, Belenotti P, de Saint-Martin L, Quemeneur T, Huart A, Bonnet F. Management of noninfectious mixed cryoglobulinemia vasculitis: data from 242 cases included in the CryoVas survey. Blood. 2012;119:5996-6004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Khan A, Tareen AM, Ikram A, Rahman H, Wadood A, Qasim M, Khan K. Prevalence of HCV among the young male blood donors of Quetta region of Balochistan, Pakistan. Virol J. 2013;10:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Ashfaq UA, Javed T, Rehman S, Nawaz Z, Riazuddin S. An overview of HCV molecular biology, replication and immune responses. Virol J. 2011;8:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Anis S, Muzaffar R, Ahmed E, Ali S, Nadir A, Naqvi A, Rizvi AH. Cryoglobulinaemia and autoimmune markers in hepatitis C virus infected patients on renal replacement therapy. J Pak Med Assoc. 2007;57:225-229. [PubMed] |
| 34. | Foessel L, Besancenot JF, Blaison G, Magy-Bertrand N, Jaussaud R, Etienne Y, Maurier F, Audia S, Martin T. Clinical spectrum, treatment, and outcome of patients with type II mixed cryoglobulinemia without evidence of hepatitis C infection. J Rheumatol. 2011;38:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Tervaert JW, Van Paassen P, Damoiseaux J. Type II cryoglobulinemia is not associated with hepatitis C infection: the Dutch experience. Ann N Y Acad Sci. 2007;1107:251-258. [PubMed] |
| 36. | Levo Y, Gorevic PD, Kassab HJ, Tobias H, Franklin EC. Liver involvement in the syndrome of mixed cryoglobulinemia. Ann Intern Med. 1977;87:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Cohen RA, Bayliss G, Crispin JC, Kane-Wanger GF, Van Beek CA, Kyttaris VC, Avalos I, Yu CY, Tsokos GC, Stillman IE. T cells and in situ cryoglobulin deposition in the pathogenesis of lupus nephritis. Clin Immunol. 2008;128:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Takeda A, Ootsuka Y, Suzuki T, Yamauchi Y, Tsujita M, Kawaguchi T, Horike K, Oikawa T, Goto N, Nagasaka T. A case report of recurrence of mixed cryoglobulinemic glomerulonephritis in a renal transplant recipient. Clin Transplant. 2010;24 Suppl 22:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Tsuboi N, Ichinose M, Kawamura T, Joh K, Utsunomiya Y, Hosoya T. Rapidly progressive cryoglobulinemic glomerulonephritis. Clin Exp Nephrol. 2010;14:492-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Martina MN, Solé M, Massó E, Pérez N, Campistol JM, Quintana LF. Mixed cryoglobulinaemia not related to hepatitis C virus, mesangiocapillary glomerulonephritis and lymphoplasmocytic lymphoma. Nefrologia. 2011;31:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 41. | Hirt-Minkowski P, Trendelenburg M, Gröschl I, Fischer A, Heijnen I, Schifferli JA. A trial of complement inhibition in a patient with cryoglobulin-induced glomerulonephritis. Case Rep Nephrol Urol. 2012;2:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Okura T, Jotoku M, Miyoshi K, Enomoto D, Kurata M, Irita J, Nagao T, Ohtsuka T, Higaki J. Case of membranoproliferative glomerulonephritis due to essential cryoglobulinemia without hepatitis C virus infection. Geriatr Gerontol Int. 2009;9:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Sargur R, White P, Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem. 2010;47:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Motyckova G, Murali M. Laboratory testing for cryoglobulins. Am J Hematol. 2011;86:500-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Vermeersch P, Gijbels K, Mariën G, Lunn R, Egner W, White P, Bossuyt X. A critical appraisal of current practice in the detection, analysis, and reporting of cryoglobulins. Clin Chem. 2008;54:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Hervier B, Rimbert M, Colonna F, Hamidou MA, Audrain M. Clinical significance of anti-Ro/SSA-52 kDa antibodies: a retrospective monocentric study. Rheumatology (Oxford). 2009;48:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Weiner SM, Prasauskas V, Lebrecht D, Weber S, Peter HH, Vaith P. Occurrence of C-reactive protein in cryoglobulins. Clin Exp Immunol. 2001;125:316-322. [PubMed] |
| 48. | Okazaki T, Nagai T, Kanno T. Gel diffusion procedure for the detection of cryoglobulins in serum. Clin Chem. 1998;44:1558-1559. [PubMed] |
| 49. | Chan AO, Lau JS, Chan CH, Shek CC. Cryoglobulinaemia: clinical and laboratory perspectives. Hong Kong Med J. 2008;14:55-59. [PubMed] |
| 50. | Müller RB, Vogt B, Winkler S, Muñoz LE, Franz S, Kern P, Maihöfner C, Sheriff A, von Kempis J, Schett G. Detection of low level cryoglobulins by flow cytometry. Cytometry A. 2012;81:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 270] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 52. | Sanchez AP, Cunard R, Ward DM. The selective therapeutic apheresis procedures. J Clin Apher. 2013;28:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4416] [Cited by in RCA: 4251] [Article Influence: 354.3] [Reference Citation Analysis (0)] |
| 54. | Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4-13. [PubMed] |