Published online Mar 16, 2014. doi: 10.12998/wjcc.v2.i3.78
Revised: January 7, 2014
Accepted: January 17, 2014
Published online: March 16, 2014
Processing time: 131 Days and 18.2 Hours
Stroke is an important cause of death and disability in adults. However, effective treatments for patients with acute ischemic stroke are limited. Intravenous recombinant tissue plasminogen activator (iv rtPA) within 4.5 h after onset has been approved as a standard treatment for patients with acute ischemic stroke. However, due to time constraints, less than one percent of acute ischemic stroke patients in Thailand are able to obtain iv rtPA. Although endovascular interventional therapy has not yet been approved as standard treatment in acute ischemic stroke, it is the one of the potentially effective treatment options. There are several reliable methods of endovascular therapy for acute ischemic stroke patients. Endovascular interventional therapy has rarely been done in Thailand. We report seven patients with successful recanalization after endovascular treatment in acute large vessel stroke from a single stroke center in Thailand. Patient screening and selection with multimodal imaging protocol and multimodality methods of endovascular interventional therapy are described.
Core tip: We report seven patients with successful recanalization after endovascular treatment in acute large vessel stroke from a single stroke center in Thailand. Patient screening and selection with multimodal imaging protocol and multimodality methods of endovascular interventional therapy are described.
- Citation: Jongsathapongpan A, Raumthanthong A, Muengtaweepongsa S. Successful recanalization with multimodality endovascular interventional therapy in acute ischemic stroke. World J Clin Cases 2014; 2(3): 78-85
- URL: https://www.wjgnet.com/2307-8960/full/v2/i3/78.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i3.78
Stroke is the leading cause of adult disability, particularly in the elderly, and remains the third most common cause of death in the developing world, as well as in Thailand[1,2]. Despite improving the quality of stroke management, morbidity and mortality related to stroke remain significant[3]. Intravenous recombinant tissue plasminogen activator (iv rtPA) is a standard treatment for patients with acute ischemic stroke[4]. The NINDS study shows that iv rtPA given within 3 h of stroke onset improves the modified Rankin Scale (mRS) at 90 d[5]. The recent ECASS3 trial expands indication of intravenous rtPA to 4.5 h[6]. Clinical benefit from iv rtPA to Thai stroke patients has been shown in studies[7,8]. However, most stroke patients are still not able to get iv rtPA due to delayed arrival and tight exclusion criteria[9].
Identification of ischemic penumbra with diffusion-perfusion mismatch by magnetic resonance imaging (MRI) may have a role in patient selection for further treatment in acute ischemic stroke[10,11]. However, the benefit on clinical outcomes of this imaging selection for endovascular treatment in patients with acute ischemic stroke is still controversial[12].
Intra-arterial thrombolysis is a viable option to some patients who arrive after the 3 h[13]. The PROACT II trial showed that recanalization rate and functional outcomes are better with intra-arterial thrombolysis[14]. Mechanical thromboembolectomy in acute ischemic stroke has received intense interest in recent years. The multi MERCI trial shows that clot removal with the device, which can be done up to 8 h after stroke onset, raises the recanalization rate up to 60%[15]. The Penumbra Pivotal trial shows that continuous thrombus aspiration with a Penumbra catheter can improve the recanalization rate to more than 80%[16]. The SWIFT trial shows that clot extraction with the Solitaire device in large vessel occlusion, including internal carotid artery (ICA), middle cerebral artery part 1 (M1), middle cerebral artery part 2 (M2) and basilar artery (BA), also provides a recanalization rate of up to 80%[17]. Unfortunately, the two most recent trials published in a landmark journal do not show any benefit in functional outcomes from endovascular treatment in acute ischemic stroke[18,19].
In Thailand, endovascular interventional therapy rarely has been performed in patients with acute ischemic stroke. Intra-arterial recombinant tissue plasminogen activator (ia rtPA) is an option in some medical centers. Imaging selection is also optional for decision making in some centers. Recently, Solitaire and Penumbra devices have been available for commercial use. We report our initial experiences with these procedures.
Phyathai 2 is a private hospital located in central of Bangkok. This 200 bed hospital provides 20 intensive care unit beds for medical intensive conditions, including acute ischemic stroke. The medical records of patients who received endovascular interventional therapy for acute large vessel occlusion (ICA, M1, M2 and BA) in Phyathai 2 Hospital during February 2010 to January 2013 were reviewed.
Acute ischemic stroke patients who were not eligible for iv rtPA or who still had significant deficits after iv rtPA were evaluated by the stroke neurologist (SM). A stroke interventional team (Jongsathapongpan A, Raumthanthong A) was alerted. Multimodal MRI (conventional MRI with MRA and MR perfusion) was done on an emergency basis. If the diffusion-perfusion mismatch was more than 20%, the patient would be transferred to the catheterization lab for endovascular treatment. The anesthesiologist was standing by in the catheterization lab.
The right femoral artery was cannulated with an 8 F sheath. Selective angiography of the carotid or vertebral artery was done with a 5 F Simmon 1 or a 5 F JR4 catheter. The aortic arch angiogram and 4 vessel DSA were not routinely performed. If an occluded artery was confirmed, a 6 F 90 cm sheath was placed as far as a distal cervical ICA or a distal V2 segment. A 018 microcatheter was advanced over the guidewire to the occlusion site. Low dose ia rtPA (less than 5 mg) was given. If no clot lysis was seen, continuous clot aspiration using a Penumbra device or clot extraction with a Solitaire device was performed.
We identified 7 cases. Age ranged from 56-87 years. National Institutes of Health Stroke Scale (NIHSS) ranged from 9-30. Multimodal MRI was done in 6 of 7 cases (86%). There were 2 patients with ICA occlusion, 2 with middle cerebral artery (MCA) occlusion and 3 with BA occlusion. Carotid stenting was performed in one case. ia rtPA, mechanical thrombectomy and combined treatment were done in 4, 5 and 3 cases, respectively. Solitaire and Penumbra devices were used in 2 and 3 cases, respectively. Only 1 patient received intervention after intravenous thrombolysis. Case presentation and treatment are summarized in Table 1.
| Sex | Age | Location | NIHSS | Onset (h) | AF | iv rtPA (mg) | ia rtPA(mg) | Solitaire | Penumbra | Carotid stent | Final mRS | Any ICH | sICH | |
| 1 | F | 56 | ICA | 20 | 3 | - | 59 | 8 | Y | - | - | 2 | N | N |
| 2 | F | 80 | BA | NA | 5.5 | Y | - | - | - | 032” | - | 4 | N | N |
| 3 | M | 64 | ICA, M1 | 10 | 5.5 | - | - | - | - | - | Wall stent | 1 | N | N |
| 4 | M | 81 | BA | 30 | 13 | Y | - | - | Y | - | - | 0 | N | N |
| 5 | M | 61 | M1 | 9 | 6 | - | - | 5 | - | - | - | 0 | Y | N |
| 6 | F | 87 | BA | NA | 5 | Y | - | 5 | - | 041” | - | 4 | N | N |
| 7 | M | 70 | M1 | NA | 5 | - | - | 5 | - | 041” | - | 2 | Y | N |
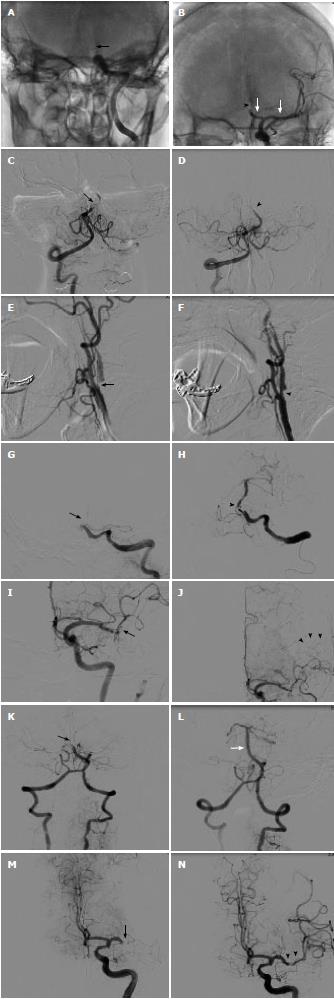
Left distal ICA occlusion opened withiartPA and Solitaire device: A 56-year-old female presented with right hemiparesis and aphasia. She arrived at hospital 1 h after onset. Emergency computed tomography (CT) brain showed cord sign in left MCA and distal ICA. Initial NIHSS was 20. Electrocardiography (EKG) showed normal sinus rhythm. Echocardiography showed no intracardiac thrombus. iv rtPA was given 90 min after onset. No neurological improvement was noted. Two hours after iv rtPA, angiography was done. We found distal ICA occlusion. Balloon inflation with a 2.0 mm × 15 mm coronary balloon was attempted without success. Eight milligrams of ia rtPA was infused. No clot lysis was seen. Then, a 4.0 × 15 Solitaire device was deployed 5 h after onset. Immediate angiography showed thrombolysis in cerebral infarction (TICI) 2 flow. The Solitaire was slowly pulled back and a large thrombus was removed. Residual stenosis of mid M1 persisted but it resolved after 1 mg of nimodipine. Final angiography showed TICI 3 in M1 and anterior cerebral artery part 1 (A1). Occlusion of anterior cerebral artery part 2 (A2) was noted. No further intervention was attempted. Six months after the procedure, mRS was 2 (Figure 1A and B).
BA occlusion opened withiartPA and Penumbra device: An 80-year-old female presented with alteration of consciousness. She had hypertension and chronic AF. Warfarin had been discontinued during the last month for unknown reasons. Immediate CT brain showed hyperdense basilar artery. MRI and MRA brain revealed small right cerebellar infarction and occlusion of mid basilar artery. Patent bilateral fetal type posterior cerebral artery was noted. Echocardiography showed no intracardiac thrombus. She was transferred to the catheterization lab 5.5 h after onset. Angiogram showed near occlusion of mid BA. Continuous thrombus aspiration with a 032 Penumbra catheter was done. TICI 3 was seen from proximal to mid basilar artery and bilateral superior cerebellar artery. Occluded distal basilar artery could not be opened. MRI brain on the next day showed bilateral superior cerebellar infarction. No intracranial hemorrhage was seen. After 3 mo mRS was only 4. Four months later, she suffered from a left MCA stroke despite dabigatran maintenance. No thrombolytic drug was given because of late presentation (Figure 1C and D).
Tandem ostial left ICA and distal M1 occlusion opened with carotid stent: A 64-year-old male was admitted for prostate surgery. Two days after the operation, he developed a right hemiparesis and dysphasia. Initial NIHSS was 10. MRI and MRA brain showed small left MCA infarction and severe ostial left ICA stenosis. Because of symptom fluctuation, iv rtPA was not given. Endovascular treatment was done because of a large diffusion-perfusion mismatch (> 20%). Angiography was done 5.5 h after onset. Critical ostial ICA stenosis and occlusion of supraclinoid ICA were seen. After deployment of a distal protection device, carotid stenting was done using 7.0 mm × 30 mm WALLSTENT™. Angiogram showed good flow of left ICA. Occluded distal M1 was noted. No further intervention was attempted because of good collateral flow. Two days after the procedure, NIHSS was 1 and mRS was 1 (Figure 1E and F).
Basilar artery occlusion opened with Solitaire device: An 81-year-old male presented with left hemiparesis. He arrived at hospital 7 h after onset. He had hypertension, dyslipidemia and chronic atrial fibrillation. Echocardiography revealed no intracardiac thrombus. MRI and MRA brain showed small right cerebellar infarction and mid basilar artery occlusion. He was transferred to the catheterization lab 13 h after onset. Angiography showed tortuous left vertebral artery and occluded proximal BA. We failed to advance a 5 F hydrophilic catheter over the left vertebral artery. Then, a homemade 90 cm shortened JR 7 F guiding catheter was placed at the proximal vertebral artery. A 4.0 mm × 15 mm Solitaire was deployed at the basilar artery. After thrombus extraction, TICI 3 flow of basilar was noted. Some residual thrombus remained in the basilar artery. No further intervention was attempted. He regained full consciousness the next day. Final NIHSS was 1 and mRS was 0 (Figure 1G and H).
Left distal M1 occlusion opened withiartPA: A 61-year-old male presented with right arm weakness and dysphasia. He arrived at hospital 1 h after onset. Initial NIHSS was 9. EKG was sinus rhythm. Echocardiography showed no intracardiac thrombus. MRI and MRA brain revealed small infarction in the left MCA area and left distal M1 occlusion. A large diffusion-perfusion mismatch was seen. He was transferred to the catheterization lab 4 h after onset. Angiography showed occlusion of superior M2 and slowed flow in the inferior M2 branch. Good pial collateral flow to the left superior M2 area was seen. Five milligrams of ia rtPA was given. TICI 3 flow of M1 and inferior M2 was noted. The superior M2 branch was still occluded. No further intervention was attempted. CT brain on the next day showed small spot hemorrhage in the left temporal lobe and small infarction of the left corona radiata. Right hemiparesis improved after the procedure. Three months later, he had only mild dysphasia and mRS was 0 (Figure 1I and J).
BA occlusion opened withiartPA and Penumbra device: An 87-year-old female patient was referred to our hospital because of loss of consciousness. Initial CT scan showed no significant hypodense area. EKG showed atrial fibrillation. MRI and MRA brain showed left pontine infarction and small bilateral cerebellar infarction. She was transferred to the catheterization lab 5 h after onset. Angiogram showed occlusion of distal BA. ia rtPA 5 mg was given without improvement. Four minutes of continuous thrombus aspiration with a Penumbra 041 catheter was done. Complete clot removal was seen. FU CT brain on the next day showed no intracranial hemorrhage but a new right occipital lobe infarction was seen. Despite the good angiographic outcome, she only had mRS 4 on the final visit (Figure 1K and L).
Left M1 occlusion opened withiartPA and Penumbra device: A 70-year-old male patient was referred to our hospital because of stupor, right hemiplegia and aphasia. He had diabetes, hypertension and was post coronary artery bypass surgery. EKG showed normal sinus rhythm. Echocardiography showed no intracardiac thrombus. Initial CT scan showed old cerebral infarction and so iv rtPA was not given. MRI and MRA brain showed occlusion of left M1. DWI showed no acute infarction. He was transferred to the catheterization lab 5 h after onset. Angiogram showed occlusion of left distal M1. ia rtPA 5 mg was given via a Rebar microcatheter without success. Three minutes of continuous aspiration with Penumbra 041 catheter was done. Complete clot removal was seen. CT brain on the next day showed small subarachnoid hemorrhage in the left sylvian fissure. No new infarction was seen. Three months later, he had only mild weakness of the right arm and mRS was 2 (Figure 1M and N).
We described 7 cases of endovascular treatment with successful recanalization in acute ischemic stroke patients. Good outcome, defined by mRS less than 2, were found in 5 of 7 cases (71%). When mechanical thromboembolectomy devices were used, successful recanalization rate and good outcome were found in 80% and 60%, respectively, which are comparable to 81% and 25%, respectively in the PENUMBRA pivotal trial and 61% and 58%, respectively in the SWIFT trials. There was no mortality in our series, compared to 38% in the PENUMBRA pivotal trial and 17% in the SWIFT trial. In our series, intracranial hemorrhage and symptomatic intracranial hemorrhage were found in 28% and 0%, respectively, which is comparable to 28% and 11%, respectively in the PENUMBRA pivotal trial and 17% and 2%, respectively in the SWIFT trial[16,17]. In our case series, younger (less than 80 years old) patients and good collateral supply were good prognostic indicators. We observed that in patients under 80 years old, all patients had good outcome (4 of 4) and in the presence of collateral supply (case 3 and case 5) a good outcome may be achieved even if the direct flow cannot be restored.
Multimodal MRI is the most reliable study to select the patients[20,21]. Patients with a small infarct core but large diffusion-perfusion mismatch are more likely to have better outcomes[21-23]. There is evidence that multimodal CT is also able to identify the infarct core and penumbra area[21,24]. However, high dose of iodinated contrast usage during CT may be contra-indicated in some patients[25]. Application of the ASPECT score with multimodal CT may be helpful for patient selection and outcome prediction[26,27].
Intra-arterial thrombolysis is one of preferred treatments in some centers[13,28]. Based on the PROACT trial, patency rate (TICI 2, 3) was 66% and mRS less than 2 at the 90th day was 40%, but in our case series, no clot lysis was found in any case[14]. It might be due to the limited dose of rtPA we used (less than 5 mg) and that the waiting time was too short (average 10-20 min). Anyway, we believed that ia rtPA still had a role in some patients, such as patients with small thrombus burden and patients with very tortuous neck arteries. However, it is likely that the role of ia rtPA will be surpassed by high efficacy mechanical devices in the near future[29].
Recently, mechanical thrombectomy devices in acute stroke have received intense interest[29,30]. High patency rate (61%-86%) and improved clinical outcome were reported in the SWIFT, PENUMBRA and TREVO trials[16,17,31]. However, individual devices may have their own technical issues. A stent based device, using a dragging method, may cause thrombus embolization into new territory. The possible solutions for this problem are to allow the device to “ingest” the thrombus for few minutes, to slowly pull back (1 cm/min) and to add aspiration force through the sheath or guide catheter. The advantage points of stent based devices are small delivery profile and speed of recanalization[32].
Continuous thrombus aspiration using a Penumbra device has one inherited problem, that is “profile”[33]. Because of a larger profile, it may require delivery in triaxial fashion over the guidewire and microcatheter. The strong advantage of a Penumbra device is more complete clot removal and less embolization into new territory[33]. This could benefit the patients with large thrombus burden and in the situation with residual thrombus after the dragging method. The aspiration method, compared to the dragging method, is perceived to result in less vessel trauma. Clinical trials reported no difference in intracranial hemorrhage, compared to the Solitaire device[29,30,33].
We plan to reduce time to recanalization in our center. Focused stroke MRI protocol may shorten it by a few minutes in this critical condition. Using multimodal CT instead of MRI may also be a time saver. An interventionist should be available 24/7. Activation of the interventional team during the imaging study is crucial. Using mechanical thromboembolectomy as a first line treatment, instead of intra-arterial thrombolysis, should be of benefit.
The authors report seven patients with successful recanalization after endovascular treatment in acute large vessel stroke from a single stroke center in Thailand.
There were 2 patients with internal carotid artery occlusion, 2 with middle cerebral artery occlusion and 3 with basilar artery (BA) occlusion.
Multimodal magnetic resonance imaging was done in 6 of 7 cases (86%).
Carotid stenting was performed in one case. Intra-arterial recombinant tissue plasminogen activator, mechanical thrombectomy and combined treatment were done in 4, 5 and 3 cases, respectively. Solitaire and Penumbra devices were used in 2 and 3 cases, respectively.
Multimodal magnetic resonance imaging (MRI) is the most reliable study to select the patients.
Focused stroke MRI protocol may reduce time by a few minutes in this critical condition. Using multimodal computer tomography instead of MRI may also be a time saver.
The manuscript is a nicely written collection of 7 cases of acute ischemic stroke that were treated with various endovascular techniques. The report is worthy of being published.
P- Reviewers: Rafay M, Sharma V, Zhang M S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3532] [Cited by in RCA: 3492] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 2. | Hanchaiphiboolkul S, Poungvarin N, Nidhinandana S, Suwanwela NC, Puthkhao P, Towanabut S, Tantirittisak T, Suwantamee J, Samsen M. Prevalence of stroke and stroke risk factors in Thailand: Thai Epidemiologic Stroke (TES) Study. J Med Assoc Thai. 2011;94:427-436. [PubMed] |
| 3. | Poungvarin N. Burden of stroke in Thailand. Int J Stroke. 2007;2:127-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-1711. [PubMed] |
| 5. | Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587. [PubMed] |
| 6. | Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. [PubMed] |
| 7. | Muengtaweepongsa S, Dharmasaroja P, Kummark U. Outcomes of intravenous thrombolytic therapy for acute ischemic stroke with an integrated acute stroke referral network: initial experience of a community-based hospital in a developing country. J Stroke Cerebrovasc Dis. 2012;21:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Suwanwela NC, Phanthumchinda K, Likitjaroen Y. Thrombolytic therapy in acute ischemic stroke in Asia: The first prospective evaluation. Clin Neurol Neurosurg. 2006;108:549-552. [PubMed] |
| 9. | Kasner SE, Gorelick PB. Prevention and Treatment of Ischemic Stroke: Blue Books of Practical Neurology Series. Oxford: Butterworth-Heinemann 2004; 267-281. |
| 10. | Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 764] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 11. | Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 914] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 12. | Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1016] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 13. | Nguyen TN, Babikian VL, Romero R, Pikula A, Kase CS, Jovin TG, Norbash AM. Intra-arterial treatment methods in acute stroke therapy. Front Neurol. 2011;2:9. [PubMed] |
| 14. | Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 1962] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 15. | Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 884] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 16. | Penumbra Pivotal Stroke Trial I. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 701] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 17. | Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1000] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 18. | Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 940] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 19. | Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1363] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 20. | Chaturvedi S, Selim M. Multimodal imaging for acute stroke: when is it worth it? Neurology. 2013;81:608-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Fisher M, Albers GW. Advanced imaging to extend the therapeutic time window of acute ischemic stroke. Ann Neurol. 2013;73:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, Wilder MJ, Lutsep HL, Czartoski TJ, Bernstein RA. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 610] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 23. | Lemmens R, Mlynash M, Straka M, Kemp S, Bammer R, Marks MP, Albers GW, Lansberg MG. Comparison of the response to endovascular reperfusion in relation to site of arterial occlusion. Neurology. 2013;81:614-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 25. | Tarlov N, Nien YL, Zaidat OO, Nguyen TN. Periprocedural management of acute ischemic stroke intervention. Neurology. 2012;79:S182-S191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Kent DM, Hill MD, Ruthazer R, Coutts SB, Demchuk AM, Dzialowski I, Wunderlich O, von Kummer R. “Clinical-CT mismatch” and the response to systemic thrombolytic therapy in acute ischemic stroke. Stroke. 2005;36:1695-1699. [PubMed] |
| 27. | Yaghi S, Bianchi N, Amole A, Hinduja A. ASPECTS is a predictor of favorable CT perfusion in acute ischemic stroke. J Neuroradiol. 2013;Epub ahead of print. [PubMed] |
| 28. | Kirmani JF, Alkawi A, Panezai S, Gizzi M. Advances in thrombolytics for treatment of acute ischemic stroke. Neurology. 2012;79:S119-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Taqi MA, Vora N, Callison RC, Lin R, Wolfe TJ. Past, present, and future of endovascular stroke therapies. Neurology. 2012;79:S213-S220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Hennerici MG, Kern R, Szabo K. Non-pharmacological strategies for the treatment of acute ischaemic stroke. Lancet Neurol. 2013;12:572-584. [PubMed] |
| 31. | Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 865] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 32. | Novakovic RL, Toth G, Narayanan S, Zaidat OO. Retrievable stents, “stentrievers,” for endovascular acute ischemic stroke therapy. Neurology. 2012;79:S148-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Hussain SI, Zaidat OO, Fitzsimmons BF. The Penumbra system for mechanical thrombectomy in endovascular acute ischemic stroke therapy. Neurology. 2012;79:S135-S141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |