Published online Mar 16, 2014. doi: 10.12998/wjcc.v2.i3.62
Revised: December 10, 2013
Accepted: January 15, 2014
Published online: March 16, 2014
Processing time: 128 Days and 20.1 Hours
The case of a 32-year-old black woman of African descent who suffered from repeated episodes of acute pancreatitis, initially triggered when flying on airplanes, is reported. She did not drink alcohol or smoke. Genetic analysis was negative for cationic trypsinogen, serine protease inhibitor Kazal type 1 and chymotrypsin C. However, hemoglobin F was elevated. Sequencing of the thalassemia gene revealed a novel alteration in the 5’ region indicative of a functional abnormality of the molecule. Sequencing the cystic fibrosis transmembrane conductance regulator (CFTR) gene revealed a heterozygote sequence variant. The combination of a hemoglobin gene mutation known for thalassemia in conjunction with the hitherto undescribed CFTR mutation is suggested to pave the road for initial and repetitive pancreatitis attacks. This will be discussed.
Core tip: This is a discussion case with two genetic alterations, one in a pancreatitis-related gene and one in an unrelated gene that might influence the oxygenation in the pancreas.
- Citation: Löhr JM, Haas S. Can a polymorphism in the thalassemia gene and a heterozygote CFTR mutation cause acute pancreatitis? World J Clin Cases 2014; 2(3): 62-66
- URL: https://www.wjgnet.com/2307-8960/full/v2/i3/62.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i3.62
Within the group of younger patients suffering from recurrent episodes of acute pancreatitis, those not drinking alcohol impose a special diagnostic challenge. Many of these patients, who were coined to suffer from idiopathic pancreatitis even without a family history, may have mutations in the genes known to be associated with this disease[1]. Among genes identified to convey the risk of developing pancreatitis are cationic trypsinogen (PRSS1)[2], serine protease inhibitor Kazal type 1 (SPINK1)[3], chymotrypsin C (CTRC)[4] and cystic fibrosis transmembrane conductance regulator (CFTR)[5], whereas mutations in the anionic trypsinogen gene[6] appear to be protective. Some who may be negative for genetic factors may have anatomical abnormalities, such as pancreas divisum or other branching disorders[7]. Still then, a small subgroup is left with no identifiable reason. Those may suffer from rare metabolic conditions or syndromes, however, normally present with other symptoms indicative of the underlying disease. Beyond those conditions, there are rather exotic reasons for suffering from acute pancreatitis. We here report on the combination of two hitherto undescribed mutations in both an extrapancreatic and a pancreatic gene that might explain the repetitive attacks of pancreatitis.
The 31-year-old black woman, who was brought up in Kenya but had been living in Germany for the last 8 years, presented with repeated episodes of acute abdominal pain which started in the middle epigastric region and would eventually radiate into the back, suggestive of acute pancreatitis. These episodes initially occurred after flying on regular commercial aircrafts. She was hospitalized eight years prior to this attack when 23 years old for cystitis and pyelonephritis when a diagnosis of acute pancreatitis was established via computer tomography. At that time, she also suffered from iron-deficient anemia [hemoglobin (Hb) 10.7 g/dL, mean corpuscular volume 69.4 μm, iron 28 mg/dL, ferritin 23 ng/mL].
Abdominal ultrasound and computed axial tomography scan were reported to be normal; endoscopic retrograde cholangiopancreatography showed a slightly irregular main pancreatic duct (grade 0); however, no pancreas divisum and no papillary abnormalities. No overt gallstones, microlithiasis or sludge was present. After the last episode, when the diagnosis of acute pancreatitis was established by laboratory tests (elevated serum amylase and lipase) and abdominal ultrasound (swollen pancreas), the patient was referred to our outpatient pancreas clinic about one week after the last episode.
The patient denied any abdominal pain or discomfort, fever or occasional night sweating. Appetite was normal. Bowel movements were reported to be regular with normal stool consistency and she reported no weight loss. There was no family history of pancreatitis or unclear abdominal pain. The patient denied any alcohol intake, smoking or experience with other drugs. There were no known allergies, prescription medicine or over-the-counter drugs. The patient is a graduate student.
The patient was in no apparent distress. The physical exam was completely normal, vital signs stable and specifically, the abdomen was not tender. Bowel sounds were normal.
In the blood, amylase and lipase were elevated (Table 1). All other parameters were within normal limits (Table 1). Infectious pathogens known to be associated with pancreatitis (cytomegalovirus, Epstein-Barr virus, mumps, adenovirus, varicella zoster virus, rubella, human immunodeficiency syndrome, Coxsackie, Legionella, leptospira, echinococcus, filariasis, fasciolosis, toxocara, bilharzia) could be excluded by serology. She did not have and had no history of malaria. Autoantibodies against nuclei, mitochondria and smooth muscle were negative, as were rheumatoid factors. All immunoglobulins, especially total immunoglobulin G (IgG) and IgG4, were within normal limits. Fecal elastase-1 (985 μg/g) and fecal chymotrypsin (14.7 U/g) were well within the normal range.
| Parameter | Unit | Value | Range |
| WBC | 10E9/L | 4.5 | 3.6-11.0 |
| RBC | 10E12/L | 4.73 | 3.8-5.2 |
| Hb | g/dL | 14.8 | 12-16 |
| Hct | 43.9 | 35%-47% | |
| MCV | fL | 92.9 | 80-100 |
| Na | mmol/L | 139 | 135-144 |
| K | mmol/L | 4.28 | 3.5-5.2 |
| Ca | mol/L | 2.18 | 2.25-2.60 |
| Albumin | g/L | 36.4 | 35-52 |
| Blood glucose | mg/dL | 74 | 70-115 |
| Bilirubin (total) | mg/dL | 0.31 | 0.2-1.4 |
| Uric acid | mg/dL | 3.2 | 2.5-5.7 |
| Cholesterol | mg/dL | 173 | 130–260 |
| Triglycerides | mg/dL | 36 | < 200 |
| BUN | mg/dL | 18.8 | 16.7-45.8 |
| Creatinine | mg/dL | 0.7 | 0.6-1.1 |
| Alkaline phosphatase | U/L | 80 | 55-160 |
| γGT | U/L | 9 | 4-18 |
| GPT/ASAT | U/L | 5 | 5-19 |
| GOT/ALAT | U/L | 13 | 5-15 |
| Cholinesterase | U/L | 4238 | 2800-7400 |
| HbA1C | 1.51 | 1.2-4.6 | |
| Zinc | mmol/L | 34 | 16-50 |
| Amylase | U/L | 183 | 25–115 |
| Lipase | U/L | 202 | 114-286 |
| Chymotrypsin | U/g | 14.7 | > 6 |
| Elastase-1 | μg/g | 985 | > 200 |
| HbF | 0.92 | < 0.5% | |
| HbA | 92.73 | 87%-94% | |
| HbA2 | 2.14 | 1.6%-3.1% | |
| Anomalous Hb | Not detectable | ||
| ALA | μmol/d | 24 | 2-49 |
| Porphobilinogen | μmol/d | 6.6 | 0.5-7.5 |
| Total porphyrines | μg/d | 41.7 | < 145 |
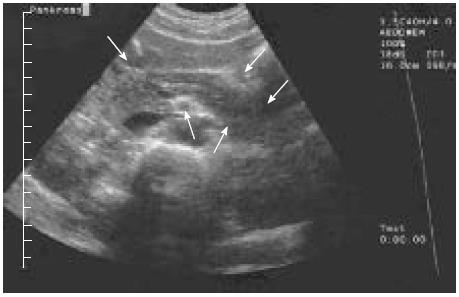
The pancreas appeared almost normal on ultrasound. Notably, the echogenicity was slightly elevated towards a so-called “white pancreas”[8] (Figure 1). There was a normal arterial blood supply to the pancreas. As the patient was slender, the transabdominal ultrasound had a good visibility: no gallstones, sludge or microlithiasis could be detected in the gallbladder or the bile duct all the way down into the pancreas.
Since the history of present illness together with the ethnic background of the patient was pressing, we performed further analysis with regard to a possible hemoglobinopathy. The ordinary complete blood count with blood smear was normal.
Hemoglobin F was slightly elevated at 0.92%, suggesting a “hereditary persistence of fetal hemoglobin” (HPFH). Molecular analysis of the hemoglobins resulted in the finding of a heterozygous transition at -158 C → T within the 5’ non-coding region of the Ggamma globulin gene (HPFH Swiss)[9,10]. Sequencing of the Agamma globulin gene did not reveal any abnormalities. Other genetic abnormalities associated with HPFH [HPFH-1 (black), HPFH-2 (Ghana), HPFH-3 (Indian), HPFH-7 (Kenya)] could not be detected. Sequencing of the β-thalassemia gene revealed no abnormalities. Sequencing the CFTR gene revealed a heterozygote sequence variant (c.2882T>C; p.M961T (ATG>ACC). Sequencing of PRSS1[2], SPINK1[3] and CTRC[4] did not reveal any additional mutations in these pancreatitis genes.
During a six year follow-up, the patient did not develop any further episodes of pancreatitis. According to our recommendation, she resisted from flying.
In adolescent and young adult patients presenting with signs of pancreatitis, associations with genetic abnormalities are pressing[1]. Even in sporadic cases, mutations in the genes known to cause hereditary pancreatitis such as PRSS1 have been reported. More recently, mutations in SPINK1 and CTRC have been reported in patients with idiopathic pancreatitis. Those were all normal/negative in this patient. The earlier history of present illness with flying to and from Kenya triggering the episodes of pancreatitis made us suspicious of other mechanisms underlying the etiology of her pancreatitis. The ethnic background led to further analysis of hemoglobinopathies, which revealed elevated hemoglobin F. Elevated fetal hemoglobin is not uncommon: it has been detected in around 10% of teenage high school students[11], a few of them with abnormalities. Amongst these, the Ggamma-globulin gene (HPFH Swiss) alteration is most frequent amongst females, all of them described as of Caucasian origin[11]. Detailed studies of our young black female patient revealed this heterozygous transition at -158 C → T within the 5’ non-coding region of the Ggamma-globulin gene (HPFH Swiss) (Table 2). No other abnormalities could be detected. Notably, the patient did not suffer from clinically overt thalassemia, not having the typical mutations in the hemoglobin A (β-chain). Since this particular variant is considered to be minor[12], this could not have been expected. Nevertheless, associations with rather complex hereditary traits have been described in these hemoglobinopathies[13].
| Etiology | Parameter | Negative/normal | Positive | Finding |
| Metabolic parameters | TSH, PTH, α-AT, lipids | X | ||
| Immunological parameters | IgG/IgG4; AMA, ANA, ASMA, RF | X | ||
| Pancreatotrophic virus | Adenovirus, Coxsackie, CMV, EBV, hepatitis, HIV, measles, mumps, Rubella, VZV | X | ||
| Pancreatitis genetics-acinar | PRSS1, CTRC, SPINK1 | X | ||
| Pancreatitis genetics-ductal | CFTR | X | c.2882T > C; p.M961T (ATG > ACC) | |
| Hemoglobin | HBs | X | Elevated | |
| HPFH | Ggamma-hemoglobin gene | X | -158 C → T (HPFH Swiss) | |
| Agamma-hemoglobin gene | X |
Classical thalassemia is reported to be associated with acute pancreatitis; however, the etiology is biliary as gallstones are typical in these patients. Indeed, in a series of 43 juvenile patients with acute pancreatitis, 30 suffered from choledocholithiasis caused by thalassemia[14].
Sequencing the CFTR gene revealed a heterozygote sequence variant that has not been described previously (Table 2). The pathophysiological meaning, especially in relation to pancreatitis, is therefore unknown.
Whereas neither of these heterozygote mutations per se might have been sufficient to cause or contribute to pancreatitis, it is suggestive that the minor alterations in two genes, one pancreatic (CFTR) and one extrapancreatic (hemoglobin), might have culminated in a two-step mechanism leading to pancreatitis: impaired hemoglobin, causing a certain degree of oxygen deficiency which is known to cause pancreatitis, e.g., during iatrogenic ischemia or heart-lung machine usage, imposed on a heterozygote mutation in the CFTR gene. This two-hit hypothesis is speculative as we did not investigate the functional changes imposed by such a transition in the non-coding region in the hemoglobulin and the CFTR mutation alone or in combination.
There is only one study reporting on a small series of patients with clinically overt thalassemia, gallstones and biliary pancreatitis[14]; however, our patient did not have overt thalassemia or gallstones/sludge or microlithiasis.
The pathological oxygen saturation of hemoglobin in thalassemia has been described[15,16]. Hypoxemia and ischemia have been demonstrated to be able to induce experimental pancreatitis[17]. Microcirculatory disturbances are today considered to play an important role in the exacerbation of acute pancreatitis[18]. Reduced perfusion of the pancreatic gland leading to hypoxia and ischemia is a well-described mechanism causing[19] or worsening pancreatitis[20]. Even in sickle-cell anemia, the acute pancreatitis is considered to be caused by microvascular occlusion[21]. Conversely, hyperbaric oxygen therapy has been demonstrated to ameliorate acute pancreatitis[22]. The combination of an alteration in a gene altering oxygen saturation with a gene altering pancreatic secretion represents the rendezvous of two genes, each in itself not sufficient to induce thalassemia or pancreatitis, an extrapancreatic and an intrapancreatic. This is supported by the finding in our patient during the initial, most severe acute pancreatitis when she was also found to have anemia (Hb 10.7 g/dL). We speculate that such combinations may be responsible for a number of hitherto undefined causes of juvenile pancreatitis. This has been observed in patients with pancreas divisum with CFTR or SPINK1 mutations, also conditions that on their own might not be sufficient to cause pancreatitis[23].
We thank the patient for permission to publish her case and to conduct the genetic analyses. We thank Heiko Witt, Munich and Jonas Rosendahl, Leipzig, for performing the genetic analyses.
The case of a 32-year-old black woman of African descent who suffered from repeated episodes of acute pancreatitis, initially triggered when flying on airplanes, is reported.
Genetic analysis was negative for cationic trypsinogen, serine protease inhibitor Kazal type-1 and chymotrypsin C. However, hemoglobin F was elevated.
The combination of a hemoglobin gene mutation known for thalassemia in conjunction with the hitherto undescribed CFTR mutation is suggested to pave the road for initial and repetitive pancreatitis attacks.
Sequencing of the thalassemia gene revealed a novel alteration in the 5’ region indicative of a functional abnormality of the molecule.
Sequencing the cystic fibrosis transmembrane conductance regulator (CFTR) gene revealed a heterozygote sequence variant.
This is indeed an interesting case that needs to be published to increase awareness of more uncommon causes.
P- Reviewers: Barreto S, Drewes AM S- Editor: Gou SX L- Editor: Roemmele A E- Editor: Wu HL
| 1. | Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Amann ST, Toskes PP. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1123] [Cited by in RCA: 1028] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 3. | Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 667] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 4. | Rosendahl J, Witt H, Szmola R, Bhatia E, Ozsvári B, Landt O, Schulz HU, Gress TM, Pfützer R, Löhr M. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2008;40:78-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Malats N, Casals T, Porta M, Guarner L, Estivill X, Real FX. Cystic fibrosis transmembrane regulator (CFTR) DeltaF508 mutation and 5T allele in patients with chronic pancreatitis and exocrine pancreatic cancer. PANKRAS II Study Group. Gut. 2001;48:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Witt H, Sahin-Tóth M, Landt O, Chen JM, Kähne T, Drenth JP, Kukor Z, Szepessy E, Halangk W, Dahm S. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Dinter D, Löhr JM, Neff KW. Bifid tail of the pancreas: benign bifurcation anomaly. AJR Am J Roentgenol. 2007;189:W251-W253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Schneider K, Harms K, Fendel H. The increased echogenicity of the pancreas in infants and children: the white pancreas. Eur J Pediatr. 1987;146:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Dubart A, Testa U, Musumeci S, Vainchenker W, Beuzard Y, Henri A, Schirilo G, Romeo MA, Russo G, Rochant H. Elevated Hb F associated with beta-thalassaemia trait: haemoglobin synthesis in reticulocytes and in blood BFU-E. Scand J Haematol. 1980;25:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Indrak K, Indrakova J, Kutlar F, Pospisilova D, Sulovska I, Baysal E, Huisman TH. Compound heterozygosity for a beta zero-thalassemia (frameshift codons 38/39; -C) and a nondeletional Swiss type of HPFH (A----C at NT -110, G gamma) in a Czechoslovakian family. Ann Hematol. 1991;63:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Leonova JYe EG, Smetanina NS, Adekile AD, Efremov GD, Huisman TH. Variability in the fetal hemoglobin level of the normal adult. Am J Hematol. 1996;53:59-65. [PubMed] |
| 12. | Kohne E. Hemoglobinopathies: clinical manifestations, diagnosis, and treatment. Dtsch Arztebl Int. 2011;108:532-540. [PubMed] |
| 13. | Losekoot M, Fodde R, Gerritsen EJ, van de Kuit I, Schreuder A, Giordano PC, Vossen JM, Bernini LF. Interaction of two different disorders in the beta-globin gene cluster associated with an increased hemoglobin F production: a novel deletion type of (G) gamma + ((A) gamma delta beta)(0)-thalassemia and a delta(0)-hereditary persistence of fetal hemoglobin determinant. Blood. 1991;77:861-867. [PubMed] |
| 14. | Cosentini A, Stranieri G, Capillo S, Notarangelo L, Madonna L, Iannini S, Ferro V, Defilippo V, Defilippo RG, Rubino R. Acute pancreatitis in the paediatric age group: a personal experience. Eur Rev Med Pharmacol Sci. 2005;9:33-40. [PubMed] |
| 15. | Rachmilewitz EA, Tamari H, Liff F, Ueda Y, Nagel RL. The interaction of hemoglobin O Arab with Hb S and beta+ thalassemia among Israeli Arabs. Hum Genet. 1985;70:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Imai K, Tientadakul P, Opartkiattikul N, Luenee P, Winichagoon P, Svasti J, Fucharoen S. Detection of haemoglobin variants and inference of their functional properties using complete oxygen dissociation curve measurements. Br J Haematol. 2001;112:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford). 2006;8:264-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Sakorafas GH, Tsiotos GG, Sarr MG. Ischemia/Reperfusion-Induced pancreatitis. Dig Surg. 2000;17:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Toyama MT, Lewis MP, Kusske AM, Reber PU, Ashley SW, Reber HA. Ischaemia-reperfusion mechanisms in acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Ahmed S, Siddiqui AK, Siddiqui RK, Kimpo M, Russo L, Mattana J. Acute pancreatitis during sickle cell vaso-occlusive painful crisis. Am J Hematol. 2003;73:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Cuthbertson CM, Christophi C. Potential effects of hyperbaric oxygen therapy in acute pancreatitis. ANZ J Surg. 2006;76:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Bertin C, Pelletier AL, Vullierme MP, Bienvenu T, Rebours V, Hentic O, Maire F, Hammel P, Vilgrain V, Ruszniewski P. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol. 2012;107:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |