Published online Dec 16, 2014. doi: 10.12998/wjcc.v2.i12.883
Revised: October 30, 2014
Accepted: November 17, 2014
Published online: December 16, 2014
Processing time: 213 Days and 11.1 Hours
AIM: To investigate whether congenital lumbar spinal stenosis (CLSS) is associated with a specific degenerative changes of the lumbar spine.
METHODS: The lumbar spine magnetic resonance imaging studies of 52 subjects with CLSS and 48 control subjects were retrospectively evaluated. In each examination, the five lumbar levels were assessed for the presence or absence of circumferential or shallow annular bulges, annular tears, anterior or posterior disc herniations, epidural lipomatosis, Schmorl’s nodes, spondylolisthesis, pars defects, and stress reactions of the posterior vertebral elements.
RESULTS: Compared to control individuals, subjects with CLSS exhibited increased incidence of circumferential and shallow annular bulges, annular tears, disc herniations and spondylolisthesis (P < 0.05).
CONCLUSION: CLSS is associated with increased incidence of degenerative changes in specific osseous and soft-tissue elements of the lumbar spine.
Core tip: Congenital lumbar spinal stenosis is associated with increased incidence of degenerative changes in specific osseous and soft-tissue elements of the lumbar spine. Describing the spectrum of the respective imaging findings, this article can assist radiologists in providing more detailed magnetic resonance imaging reports.
- Citation: Soldatos T, Chalian M, Thawait S, Belzberg AJ, Eng J, Carrino JA, Chhabra A. Spectrum of magnetic resonance imaging findings in congenital lumbar spinal stenosis. World J Clin Cases 2014; 2(12): 883-887
- URL: https://www.wjgnet.com/2307-8960/full/v2/i12/883.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i12.883
Since the first reports describing congenital (or developmental) lumbar spinal stenosis (CLSS), the clinical implications of this entity have been the subject of several scientific studies[1-3]. Based on the original etiological classification described by Arnoldi et al[4], CLSS is a developmental narrowing of the spinal canal, which is secondary to a bone dysplasia. Radiographically, the respective subjects have a shorter pedicular length and as a result a smaller cross-sectional spinal canal area[5]. In usual practice, the above subjects tend to present clinically in the fourth or fifth decade of life with various neurogenic complications and relatively few radiographically evident degenerative spondylotic changes[5]. Except for scattered articles reporting radiographic and cross-sectional measurements to define CLSS, there have been no studies evaluating whether the entity is associated with degenerative changes in specific osseous and soft-tissue elements of the lumbar spine. In our practice with magnetic resonance imaging (MRI) studies of the lumbar spine, we have experienced that individuals with CLSS tend to exhibit an increased incidence of specific imaging features, including foraminal disc protrusions, as well as a particular type of annular disc bulge, in which the posterior concavity of the intervertebral discs is preserved. To validate our observations and potentially extend our understanding of CLSS from an imaging point of view, we investigated the association between CLSS and degenerative changes in various osseous and soft-tissue elements of the lumbar spine.
Institutional review board approval was granted and informed consent was waived for this HIPAA-compliant study. The MRI database of our institution was searched for examinations of the lumbar spine performed in adults of less than or equal than 50 years of age, an age limit arbitrarily employed to limit the inclusion of subjects with age-related degenerative spine changes. Two radiologists with 16 (A.C.) and 6 (T.S.) years of radiology experience, respectively, who were blinded to the original reports of the MRI studies, evaluated the examinations in consensus on a picture archiving and communication system workstation (Ultravisual, Emageon, AL, United States). All examinations were performed on 1.5T or 3.0T MR scanners and included axial and sagittal T1- and T2-weighted images, as well as sagittal STIR images of the lumbar spine.
In each study, the T2-weighted images were used to calculate the mid-sagittal spinal canal diameter and mid-sagittal thecal sac diameter at the mid-vertebral levels of all five lumbar vertebrae. Subjects with mid-sagittal spinal canal diameter of less than 14 mm on at least one level were considered as having CLSS and were included in the study group, whereas subjects with mid-sagittal spinal canal diameter of equal or greater than 14 mm at all five levels were included in the control group. Examinations were consecutively evaluated until a study group of 52 subjects and a control group of 48 subjects were formed. Patients with achondroplasia or a known history of spinal surgery, trauma, infection and/or tumor were excluded.
In each study, the average value of mid-sagittal thecal sac diameter was calculated, and thereafter, the intervertebral levels from L1-L2 to L5-S1 were evaluated for the presence or absence of (1) circumferential annular bulge, defined as generalized extension of greater than 50% of the outer boundary of the intervertebral disc beyond the border of the adjacent bone, with loss of posterior disc concavity; (2) shallow annular bulge, defined as the extension of the intervertebral disc by greater than 50% from the outer boundary of the adjacent bone, with preservation of the posterior disc concavity; (3) annular tear(s), defined as focal area(s) of increased signal intensity within the outer layer of the intervertebral disc on fluid-sensitive images; (4) uni- or bilateral foraminal disc herniation(s), defined as extension(s) of less than 50% of the outer boundary of the intervertebral disc beyond the border of the adjacent bone, centered on one or both foramina; (5) central or paracentral disc herniation, defined as extension of less than 50% of the outer boundary of the intervertebral disc beyond the border of the adjacent bone, protruding centrally or subarticularly within the spinal canal, respectively; (6) epidural lipomatosis, registered when the epidural adipose tissue assumed an anteriorly convex border and a thickness of greater than 7 mm[6]; (7) Schmorl’s node(s); (8) spondylolisthesis (antero- or retrolisthesis), registered when the vertebral body exhibited anterior or posterior displacement of equal or greater than 1 mm over the vertebral body below; (9) uni- or bilateral pars defect(s); (10) anterior disc herniation; and (11) stress reaction (increased signal intensity on fluid-sensitive images) of the posterior vertebral elements. For each of the above features, the total incidence observed throughout the five intervertebral levels was documented for each subject.
For all evaluated quantitative parameters, the difference between the study and control groups was assessed using Student’s t test, whereas sex distribution was evaluated using χ2 test. A probability level of 0.05 was accepted as statistically significant. All data were stored on a spreadsheet (Excel 2010, Microsoft, Seattle, WA, United States) and analysis was performed using a commercially available statistical package (MedCalc 8.0, Mariakerke, Belgium).
Table 1 summarizes the demographics of the study population, the incidences of the features evaluated, as well as the results of the various statistical comparisons between the study and control groups. The two groups were similar in terms of age and sex distribution. Subjects with CLSS exhibited increased incidence of circumferential and shallow annular bulges, foraminal and anterior disc herniations, annular tears, and spondylolisthesis. There was no difference between the two groups regarding the incidences of central and paracentral disc herniations, epidural lipomatosis, Schmorl’s nodes, pars defects, and stress reaction of the posterior vertebral elements.
| Parameter | Subjects with CLSS | Control subjects | P |
| Subjects | 52 | 48 | - |
| Age | 38 ± 10 | 38 ± 8 | 0.4930 |
| Sex (males/females) | 28/24 | 22/26 | 0.2742 |
| Average mid-sagittal thecal sac diameter | 1.31 ± 0.13 | 1.51 ± 0.18 | – |
| Circumferential annular bulges | 59 (1.13 ± 0.95) | 35 (0.73 ± 0.79) | 0.01161 |
| Shallow annular bulges | 80 (1.54 ± 1.06) | 47 (0.98 ± 0.93) | 0.00311 |
| Foraminal disc herniations | 31 (0.60 ± 0.82) | 13 (0.27 ± 0.54) | 0.01111 |
| Central/paracental disc herniations | 22 (0.42 ± 0.70) | 15 (0.31 ± 0.55) | 0.1917 |
| Epidural lipomatosis | 33 (0.63 ± 1.09) | 17 (0.35 ± 0.76) | 0.0701 |
| Schmorl’s nodes | 24 (0.46 ± 1.00) | 13 (0.27 ± 0.68) | 0.1352 |
| Spondylolisthesis | 53 (1.02 ± 0.96) | 29 (0.60 ± 0.71) | 0.00811 |
| Pars defects | 0 (0.00 ± 0.00) | 2 (0.04 ± 0.20) | 0.0699 |
| Annular tears | 56 (1.08 ± 1.01) | 25 (0.52 ± 0.80) | 0.00041 |
| Anterior disc herniation | 63 (1.21 ± 1.16) | 25 (1.51 ± 0.18) | < 0.00011 |
| Posterior elements stress reaction | 4 (0.08 ± 0.33) | 2 (0.04 ± 0.20) | 0.2644 |
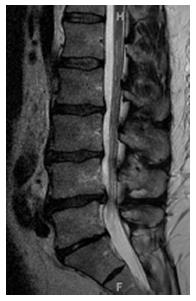
CLSS has been attributed to an abnormal anatomic development of the spinal canal. The etiology of the entity is unknown, except from some cases which are induced by achondroplasia[5,7]. CLSS differs from degenerative lumbar spinal stenosis in that the spinal canal stenosis is not limited to one or two intervertebral levels, but is uniformly distributed throughout the lumbar spine (Figure 1). As a result, the surgical treatment of CLSS commonly necessitates multi-level intervention, as opposed to degenerative lumbar spinal stenosis, which requires more focal procedures[5]. Subjects with CLSS are vulnerable to even minimal degenerative changes that compromise the already narrowed spinal canal, and tend to experience symptoms in the fourth and fifth decades of life, as opposed to patients with degenerative lumbar spinal stenosis, who demonstrate symptoms primarily after the sixth decade of life[5,7].
Although the incidence of CLSS in the general population is unknown and probably varies among different races and ethnic groups, MRI readers commonly encounter this entity in studies of the lumbar spine. CLSS has been described as early as the 1950s[3], however the imaging evaluation of this entity has been limited to delineating radiographic and cross-sectional criteria for its definition, and reporting limited degenerative spondylotic changes as a typical radiographic feature of the respective subjects. The definition of CLSS is not uniform across authors, with studies suggesting cut-off values of mid-sagittal spinal canal diameter varying between 10 and 17 mm, and not clarifying whether spinal canal narrowing needs to be documented on at least one or more spinal levels[4,7-10]. Some authors consider the cross-sectional area of the spinal canal as the criterion to define CLSS, a measurement, probably more accurate, but also time-consuming and impractical for everyday use. In addition, all previous studies have been limited in the vague description of osseous degenerative changes, and mostly using radiographs[1-3,5,7]. This study focused on specific osseous and soft tissue elements of the lumbar spine and employed a mid-sagittal spinal canal diameter of less than 14 mm on at least one mid-vertebral level to define CLSS. The latter value “summates” previous reports, has been illustrated in a large previous study by Singh et al[5] and is the one used by radiologists and orthopaedic surgeons in our institution. A recent study which compared subjects with and without CLSS by means of MRI and anteroposterior radiographs of the lumbar spine, reported that, in the CLSS cohort, global pathology and multilevel involvement with L3, L4, and L5 segments were involved more commonly and severely, whereas severe stenosis, at L1, L2, and S1 occurred infrequently. The authors also described three spinal canal morphologies in the CLSS group: (1) “flattened” canal with predominantly reduced spinal canal AP diameter; (2) spinal canal with predominantly reduced interlaminar angle; and (3) global reduction of all canal parameters[11].
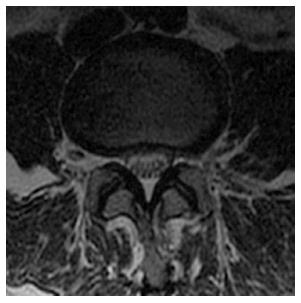
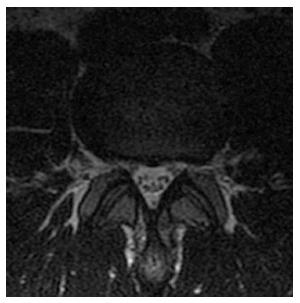
We found that young and middle-aged individuals with CLSS demonstrate increased incidence of degenerative changes in the specific osseous and soft-tissue elements of the lumbar spine. In particular, these subjects exhibit increased incidence of “shallow annular bulges”, a term which has not been previously established, but has been used in our practice to describe a particular type of disc bulge, which involves greater than 50% of the disc boundary, but does not meet the strict definition of circumferential bulge, in which a loss of the posterior disc concavity is also present (Figure 2). Shallow annular bulges preferentially narrow the neural foramina, similar to foraminal herniations. It may be speculated that, in CLSS, the thecal sac becomes less pliable due to the uniformly narrowed spinal canal, as well as less compressible due to the opposing intrinsic pressure of the cerebrospinal fluid. As a result, the thecal sac demonstrates increased resistance against posterior disc bulges or herniations in the initial stages of degenerative disc disease. In the above setting, a degenerated disc, acquiring the path of least resistance, tends to project into the foramina rather than the spinal canal. Consequently, the disc potentially maintains its posterior concavity and a shallow annular bulge is established. The above hypothesis could also explain the increased incidence of foraminal protrusions and anterior disc herniations in CLSS. The spectrum of findings is completed with increased incidence of circumferential annular bulges and spondylolisthesis (Figure 3).
Knowledge of the spectrum of MRI findings in CLSS could not only extend our understanding of the latter entity, but also assist radiologists in providing more detailed lumbar spine MRI reports. In most situations, radiologists begin the assessment of lumbar spine MRI studies from a quick evaluation of the mid-sagittal image, therefore establishing CLSS could alert readers for the presence of the aforementioned features.
This study has certain limitations. First, all studied subjects reported back pain, therefore the incidence of degenerative disc disease was probably high in this biased group, and may have affected the results. Second, all MRI examinations were evaluated in consensus by the two readers, therefore the inter-observer variability could not be estimated. Third, due to the absence of a widely accepted cut-off value of mid-sagittal canal diameter to define CLSS, we used a cut-off value that “summates” previous reports, and probably provides a good trade-off between specificity and sensitivity for detecting CLSS. However, the use of an absolute dividing line between subjects with and without CLSS is probably arbitrary, and a continuous zone between the two groups most likely exits.
In conclusion, CLSS is associated with early development of degenerative changes in specific osseous and soft-tissue elements of the lumbar spine, which could reflect altered spinal biomechanics. The spectrum of imaging findings includes the shallow annular bulge, in which the posterior concavity of the intervertebral disc is preserved. Knowledge of the above spectrum could extend our understanding of CLSS, and assist radiologists in providing more detailed lumbar spine MRI reports.
Congenital lumbar spinal stenosis (CLSS) is a developmental narrowing of the spinal canal, which is associated with early neurogenic complications and relatively few radiographically evident degenerative spondylotic changes. This article investigates the association between CLSS and degenerative changes in various osseous and soft-tissue elements of the lumbar spine.
Evaluation of subjects with CLSS by means of magnetic resonance imaging to investigate the spectrum of early degenerative changes of the lumbar spine in the respective entity.
This is the first article to describe the spectrum of early degenerative changes of the lumbar spine in subjects with CLSS.
Providing knowledge of the spectrum of early degenerative changes of the lumbar spine in subjects with CLSS this article extends our understanding of the entity and nay assist radiologists in providing more detailed MRI reports.
This is a very interesting article describing uncertain entity as congenital lumbar stenosis is.
P- Reviewer: Afzal M, Bludovsky D, Nagashima H, Panchal R S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Verbiest H. Primary stenosis of the lumbar spinal canal in adults, a new syndrome. Ned Tijdschr Geneeskd. 1950;94:2415-2433. [PubMed] |
| 2. | Verbiest H. Further reports on primary stenosis of the lumbar spinal canal in adults. Ned Tijdschr Geneeskd. 1951;95:1965-1970. [PubMed] |
| 3. | Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36-B:230-237. [PubMed] |
| 4. | Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, Gargano FP, Jacobson RE, Kirkaldy-Willis WH, Kurihara A. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976;4-5. [PubMed] |
| 5. | Singh K, Samartzis D, Vaccaro AR, Nassr A, Andersson GB, Yoon ST, Phillips FM, Goldberg EJ, An HS. Congenital lumbar spinal stenosis: a prospective, control-matched, cohort radiographic analysis. Spine J. 2005;5:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Robertson SC, Traynelis VC, Follett KA, Menezes AH. Idiopathic spinal epidural lipomatosis. Neurosurgery. 1997;41:68-74; discussion 74-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Dai LY, Ni B, Jia LS, Liu HK. Lumbar disc herniation in patients with developmental spinal stenosis. Eur Spine J. 1996;5:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Joint Surg Br. 1977;59:181-188. [PubMed] |
| 9. | Eisenstein S. The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br. 1977;59:173-180. [PubMed] |
| 10. | Vanharanta H, Korpi J, Heliövaara M, Troup JD. Radiographic measurements of lumbar spinal canal size and their relation to back mobility. Spine (Phila Pa 1976). 1985;10:461-466. [PubMed] |
| 11. | Kitab SA, Alsulaiman AM, Benzel EC. Anatomic radiological variations in developmental lumbar spinal stenosis: a prospective, control-matched comparative analysis. Spine J. 2014;14:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |