Published online Dec 16, 2014. doi: 10.12998/wjcc.v2.i12.852
Revised: July 7, 2014
Accepted: October 1, 2014
Published online: December 16, 2014
Processing time: 230 Days and 17.8 Hours
Idiopathic granulomatous mastitis is a rare chronic inflammatory lesion of the breast that can clinically and radiographically mimic breast carcinoma. The most common clinical presentation is an unilateral, discrete breast mass, nipple retraction and even a sinus formation often associated with an inflammation of the overlying skin. The etiology of idiopathic granulomatous mastitis is still obscure. Its treatment remains controversial. The cause may be the autoimmune process, infection, a chemical reaction associated with oral contraceptive pills, or even lactation. Various factors, including hormonal imbalance, autoimmunity, unknown microbiological agents, smoking and α 1-antitrypsin deficiency have been suggested to play a role in disease aetiology. In this review, causing factors in the aetiology of idiopathic granulomatous mastitis are reviewed in detail.
Core tip: Aetiology of idiopathic granulomatous mastitis has not been fully elucidated. In this article, possible aetiologic factors mentioned in the literature are discussed in detail. Additionally, ethnicity factor which is briefly mentioned previously in the literature are detailed.
- Citation: Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases 2014; 2(12): 852-858
- URL: https://www.wjgnet.com/2307-8960/full/v2/i12/852.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i12.852
Inflammatory events are frequently seen in the breast, and its can appear in a manner that clinically mimics malignancy, but they are usually benign. In addition, aetiological factors (trauma and breast-feeding) are generally identified after a detailed anamnesis. In cases where the aetiology is defined, it is easy to practice treatment algorithms starting with “limiting or removing the aetiological factor”. In cases where the aetiological factor is unknown, the diagnosis, differential diagnosis and treatment steps can become more complicated.
Since idiopathic granulomatous mastitis (IGM) was defined as a distinct clinical entity among benign breast diseases in 1972[1], it has attracted clinicians’ attention, particularly those interested in breast diseases.
The differential diagnosis of IGM with breast cancer only clinically without histopathological examination is almost impossible. Therefore, presence of some complaints which can be seen in both disease (like palpable breast mass, nipple retraction, nipple skin hyperemia, erosion and fistula formation) would be more accurate to think of the first diagnosis of malignancy. In histopathological examination, presence of granulomatous inflammation and no malignancy is require performing other tests for the aetiology. Failure various factors which may result in granulomatous reaction in the breast (tuberculosis, certain parasitic and fungal infections, Wegener’s granulomatosis, giant-cell arthritis, polyartheritis nodosum, sarcoidosis, foreign body reaction, etc.) will support the diagnosis of IGM.
In this article, we discuss factors that may play a role in the aetiology of IGM.
Idiopathic granulomatous mastitis (GM) is a rare, benign, chronic, inflammatory lesion of the breast, and its aetiology has not been fully elucidated. It was defined for the first time in 1972 by Kessler and Woollock and was described in detail in 1977 with a five-case series by Cohen[1,2] .
GM is generally divided into two main groups of specific and non-specific. The term “specific GM” refers to conditions for which the aetiological factor can be identified, whether an isolated inflammatory event only applies to the breast, or the breast is involved in a systemic inflammatory event. Nonspecific GM is also known as idiopathic granulomatous mastitis or granulomatous lobular mastitis, which generally refers to conditions that can lead to a granulomatous reaction in the breast or conditions for which the aetiological factors cannot be determined.
Chronic granulomatous inflammation constitutes 24% of all inflammatory events of the breast that are histopathologically defined[3]. All factors that lead to granulomatous inflammation in the breasts are presented in Table 1[4].
| Infectious | Mycobacterium tuberculosis |
| Blastomycosis | |
| Cryptococcosis | |
| Histoplasmosis | |
| Actinomycosis | |
| Filarial infection | |
| Corynebacterium | |
| Autoimmune process | Wegener granulomatosis |
| Giant cell arteritis | |
| Foreign body reaction | |
| Duct ectasis | Plasma cell mastitis |
| Subareolar granuloma | |
| Periductal mastitis | |
| Diabetes mellitus | |
| Sarcoidosis | |
| Fat necrosis | |
| Idiopathic |
While IGM mostly emerges in young-middle age women (third and fourth decades), the age range that has been reported in the literature (11-83 years) is considerably wider[5-7]. IGM is usually seen within a couple of years after giving birth, and the majority of patients have a history of at least one live birth and breast feeding[8]. In contrast, specific GM is frequently seen in Asian and African countries and can be detected at any age[9].
IGM may present with clinical findings that mimic the two endpoints of breast diseases such as breast abscess and breast cancer[8]. A palpable mass in the breast is the most common complaint, but nipple retraction, hyperaemia in breast skin, oedema, ulceration and fistule development during the chronic period are also potential complaints[9]. Systemic symptoms such as fever are generally not present[6]. While the incidence is the same in both breasts, the lesion is usually unilateral and cases with bilateral involvement have been reported only rarely[8,10,11].
The pathogenesis of IGM is not exactly known, but different steps occur in the disease pathogenesis. One of these steps is nonspecific lobulitis, which involves multiple lobules, and causes reactive lymphoplasmocytic infiltration. A granulomatous formation with central supurative necrosis occasionally occurs because of lobule deformation. Abscesses develop because of an increase in the number of these foci[12].
Some studies have indicated the similarity between IGM and granulomatous inflammation of the testicles or the thyroid gland when IGM was defined for the first time[1]. Considering that mechanical factors are responsible for the formation of granulomas of the thyroid gland in multifocal granulomatous thyroiditis (palpation thyroiditis), the possibility that trauma represents another stage in IGM pathogenesis should not be disregarded[12].
A process starting with non-puerperal secretion has been proposed as the most rational theory for the pathogenesis of IGM. A hormonal imbalance due to a deviation in the oestrogen-progesterone ratio or hyperprolactinemia is believed to cause this secretion and inflammation. Ductal ectasia occurs due to the intra-ductal accumulation of a protein-rich secretion. Permanent inflammation occurs following perforation of the ducti and contact between the secretion and stromal cells. The accumulation of secretion, ductal ectasia, galactoporitis (intraductal inflammation) and chronic GM are steps in the pathophysiological process. Autoimmunity against a secretion that is extravasated from the lobules is also considered to cause this event[6,13,14].
The aetiology of IGM remains unclear. Various factors, including hormonal imbalance, autoimmunity, unknown microbiological agents, smoking and α1-antitrypsin deficiency have been suggested to play a role in disease aetiology.
α1-antitrypsin deficiency
α1-antitrypsin (AAT) is a glycoprotein synthesised by hepatic cells. Similar to anti-thrombin 3, ovalalbumin and thyroid-binding globulin, AAT is a member of the serine-protease inhibitor family. Its primary function is to prevent the destructive effects of proteases secreted from activated neutrophils (proteinase 3, elastin and cathepsin G). Because AAT level is elevated during inflammation, it is also accepted as an acute phase reactant. Deficiency in AAT leads primarily to lung and liver pathologies[15]. In their case presentation in 2001, Schelfout et al[16] demonstrated AAT deficiency in a 37-year-old female patient diagnosed with IGM. According to that study, the authors did not determine any other aetiological factors, and suggested that AAT deficiency could be the aetiological factor; however, further studies were not performed.
The secretion theory has an important place in the pathophysiology of IGM. Oral contraceptives (OCS) have been considered a potential aetiological factor, as they increase breast secretion[12]. However, a significant association between OCS and IGM has not been determined. Oran et al[17] found 10 cases (10/46; 21.7%) that had a history of OCS use; Gurleyik et al[18] found eight cases (8/19, 42.1%) that had a history of OCS use; and Al-Khaffaf et al[19] found five cases (5/18, 27.7%) that had a history of OCS use. In contrast, Baslaim et al[20] reported that none of 20 patients had a history of OCS use. Bani-Hani et al[7] found that only two of 24 cases (8.3%) had a history of OCS use, and Asoglu et al[21] found that only two of 18 cases (11.1%) had a history of OCS use. In conclusion, the association between IGM and OCS use has been reported to range between 0%-42%.
Given that IGM is usually detected in women < 50 years of age, and frequently involves a recent history of birth or breast-feeding, these factors have been considered in the disease aetiology. Hormonal alterations during these processes, secretion, and inflammation have an effect on disease pathophysiology[19,21-28]. Bani-Hani et al[7] carried out a study on 24 cases, and found that four had active gestation, four had a history of birth and breast-feeding within 6 months and only two cases did not have a history of gestation. According to a study by Baslaim et al[20], all cases had a history of gestation and breast-feeding, whereas two cases were actively breast-feeding, and one case had an active gestation. Similarly, Gurleyik et al[18] determined that four of 19 cases had a history of active breast-feeding, and the remaining 15 cases had a history of breast-feeding. Moreover, Oran et al[17] reported that only three of 46 cases were nulliparous. Gautier et al[14] conducted a case series study on 11 cases and emphasised that all cases except one male case had a history of birth and lactation within the past 5 years.
While almost all studies reported a history of parity, various studies have failed to explain the timing of the parity. It is expected that cases with IGM, which is a reproductive age disorder, have a history of gestation and breast feeding, as gestation occurs between the ages of 20-40 years. In addition to the male case, cases with a wide age range (11-83 years) in the literature make it difficult to hold only gestation, birth and breast feeding responsible for the aetiology of IGM[4,5,14].
Considering the secretion theory, hyperprolactinemia has also been considered responsible for the pathogenesis of IGM, similar to other hormonal disorders[12,29,30]. In a case presentation in 1984, Rowe[29] determined co-morbid prolactinoma in an IGM case. However, future studies did not provide prolactin levels in detail. Bani-Hani et al[7] analysed prolactin levels in seven of 24 cases and found elevated prolactin levels in one patient (4.1%). Erhan et al[10] carried out a case-series study on 18 women and reported recurrence in three cases (16%), and identified hyperprolactinemia in two of these patients.
While smoking is among the factors considered in the disease aetiology, a definitive association between smoking and IGM has not yet been established. According to a study by Asoglu et al[21], 14 of 18 cases (77.8%) had a history of smoking, whereas Baslaim et al[20] reported that none of their 20 cases had a history of smoking. In addition, the smoking rate was 34.8% according to Oran et al[17], 16.7% according to Al-Khaffaf et al[19], and 50% according to Ozel et al[31].
A hypothesis that suggests an immunological basis for IGM has received considerable attention. Literature findings, including a good response to steroid and immunosuppressive treatment, patients who had recurrence after surgery showing a good response to steroid treatment, patients with extramammary involvement (such as erythema nodosum, or arthritis) and the demonstration of T-lymphocyte dominance in immunohistochemical studies support the autoimmunity hypothesis[1,2,11-14,32]. Ozel et al[31] conducted a study on eight cases and found that six were positive for rheumatoid factor (RF), and two were positive for antinuclear antibody (ANA) and anti-double stranded DNA (anti-dsDNA). In that study, surgery was the preferred treatment option for all patients, and the authors reported recurrence in two patients who were RF-, ANA- and anti-dsDNA-positive, but obtained a positive response after steroid treatment. Erhan et al[10] conducted an immunohistochemical evaluation, and determined that 14 of 18 cases had T-cell dominance, and this finding was interpreted as an autoimmune pathophysiological outcome that progressed with reactive T-cell-mediated inflammation and centrilobular granulomas against ductal damage. Furthermore, two IGM cases with erythema nodosum, one IGM case with erythema nodosum and arthritis, one IGM case with Weber-Christian disease and one IGM case with Sjögren’s syndrome have been reported in the literature[33-36]. However, cases with a co-morbid autoimmune disorder constitute only a minor fraction of all cases. In contrast to these studies that support the autoimmune hypothesis, classical serological tests, which are used for autoimmune disorders such as ANA and RF, reveal different results in patients with IGM. Asoglu et al[21] conducted a case-series study on 18 cases and determined that all cases were negative for ANA and RF. We conducted a study in our clinic to investigate the autoimmunity hypothesis for IGM aetiology, and evaluated ANA and extractable nuclear antibody levels in 26 cases, but we did not obtain results to support the autoimmunity hypothesis[37].
The normal endogenous bacteria flora of the breast is similar to the skin flora. Dominant organisms include coagulase-negative streptococci, Propiniobacterium sp. and Corynebacterium sp. These findings have been proven through nipple discharge and breast tissue cultures that were collected during mammoplasty[38]. These bacteria are considered to go deeper into the breast tissue via the ductal system[13].
Corynebacteria cause mastitis in livestock. However, these bacteria are not expected pathogens in humans[13]. These bacteria became the centre of attention in 2003, with detection of corynebacteria in 34 IGM cases by Taylor et al[39].
Corynebacteria are Gram-positive bacteria and members of the skin flora. It is hard to distinguish whether these organisms cause infection, colonisation or contamination[40]. Despite the difficulty in distinguishing outcome, it is significant to detect purulent matter in an abscess or > 104 CFU/mL dominant Corynebacterium sp.[41]. According to a study by Funke et al[42], these bacteria could be a possible factor if: (1) a Gram-positive bacillus accompanying polymorphonuclear leukocytes is present; or (2) a Corynebacterium sp. is detected in a tissue that is expected to be sterile under normal conditions.
Four different Corynebacterium species have been detected in IGM cases. Corynebacterium kroppenstedtii (C. kroppenstedtii) is the most frequently observed species, and is different from other corynebacteria due to its lipophilic nature and positive esculin test[39,40].
Taylor et al[39] conducted a study of 62 patients who were histologically diagnosed with GM, and detected Corynebacterium in 34 patients (54.8%). A comparison among the remaining 28 cases showed that fever and neutrophilia were more frequently observed in cases that were bacteria-positive, and they had more frequent fistule formation. C. kroppenstedtii was the most frequently observed species (14 patients; 41.1%) in that study.
Paviour et al[40] isolated Corynebacterium from breast tissue in 24 cases, carried out a histopathological evaluation in 12 of these cases and diagnosed nine cases with IGM. Similarly, C. kroppenstedtii was the most frequently isolated species in that study; C. amycolatum and C. tuberculostearicum were other identified species. In that study, a 3-week intravenous penicillin treatment was tested on one patient; however, when the expected benefit was not observed, the treatment was switched to doxycycline (100 mg, oral), which has better fat solubility. The authors reported that there was no need for surgery after this treatment.
Case presentations in which Corynebacterium sp. have been detected are also present in the literature[41,43,44]. A specific species was not reported in two of these studies, whereas Ang et al[41] reported that they isolated C. accolens. All three studies stated that antibiotherapy was effective for treatment.
In our clinic, we carried out a study on 45 patients with IGM and 34 bacteria using a universal DNA primer, but we did not detect positivity for any microbiological agent (unpublished data).
During our search of GM in the PubMed database (1995-2014), we searched the terms “idiopathic granulomatous mastitis”, “granulomatous lobular mastitis” and “granulomatous mastitis” and found approximately 200 articles. We hypothesised that an evaluation based on the location of the centres in which the authors worked would provide a rough estimate of the distribution of the cases. While most of these studies were case presentations, we found that larger case series frequently originated from the Mediterranean region and the developing countries in Asia. Some authors have considered that undiagnosed tuberculosis cases might lead to GM[15,45-48].
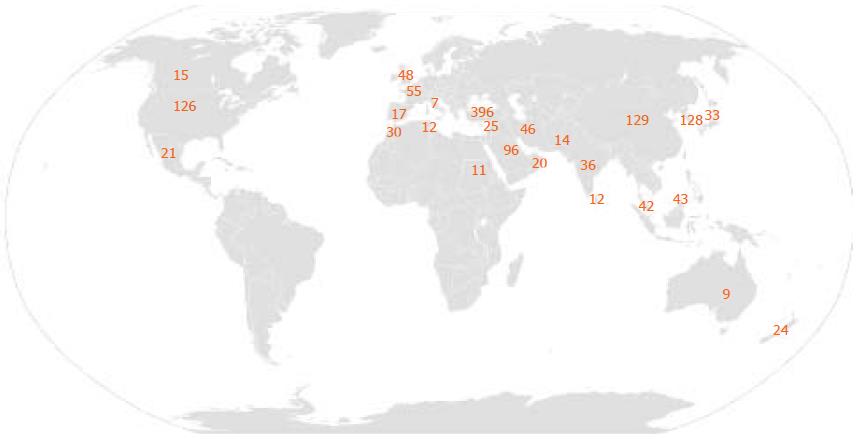
According to our search on the PubMed database, the highest number of cases has been reported in Turkey (> 200 cases). This is followed by China (129 cases) and South Korea (128 cases). France had the highest number of cases (55 cases) among European countries, and no other country exceeded 50 cases. In contrast, we found 126 cases in the United States. The total number of cases recorded per country is presented in Table 2 and Figure 1.
| > 100 cases | 20-100 cases | 5-20 cases | < 5 cases |
| Turkey: > 200 | Saudi Arabia: 96 | Spain:17 | Netherlands: 4 |
| China: 129 | France: 55 | Canada: 15 | Israel: 4 |
| South Korea: 128 | United Kingdom: 48 | Pakistan: 14 | Austria: 3 |
| United States: 126 | Iran: 46 | Sri Lanka: 12 | Belgium: 3 |
| Brunei: 43 | Tunis: 12 | Taiwan: 2 | |
| Malaysia: 42 | Sudan: 11 | Caribbean: 2 | |
| India: 36 | Australia: 9 | Peru: 1 | |
| Japan: 33 | Italy: 7 | Nigeria: 1 | |
| Morocco:30 | Kuwait: 1 | ||
| Jordan: 25 | Jamaica: 1 | ||
| New Zealand: 24 | Greece: 1 | ||
| Mexico: 21 | Norway: 1 | ||
| Oman: 20 |
According to a Centers for Disease Control and Prevention report, which was published in Morbidity and Mortality Weekly Report in 2009, seven cases were detected in Indiana between 2006 and 2008, and six of these cases were born in Mexico and had a Hispanic. According to the report, this series was the most comprehensive case series reported in the United States[49]. However, Larsen et al. published a study on 54 cases in the same year, but the authors did not evaluate ethnicity[50]. Gautier et al[15] carried out a study on 11 cases in Canada and reported that three cases were French, two cases were Canadian of French origin, two cases were Canadian of British origin, two cases were Latin American and one case was Russian. Furthermore, Omranipour et al[50] reported a series of 43 cases in Iran, Bani-Hani et al[7] reported a series of 24 cases in Jordan and Baslaim et al[20] reported a series of 20 cases in Saudi Arabia. In Turkey, different IGM series have been reported by Asoglu et al[21] (18 cases), Ozel et al[31] (8 cases), Gurleyik et al[18], Oran et al[17] (46 cases) and Altintoprak et al[37] (26 cases). These findings indicate that a previous comprehensive evaluation of ethnicity does not exist, and that more elaborate studies on this topic are required.
In conclusion, while several factors have been considered as potential aetiological factors, these factors are not ‘the primary aetiological factors, but rather “secondary factors” that can accompany the process once the primary factor triggers the event, or contribute to the acceleration’ of the ongoing process. Given that: (1) a higher number of cases are being reported from certain geographical locations; and (2) patients respond positively to steroid treatment, we believe that the “ethnicity and autoimmunity hypotheses” are the major subjects to focus on. It is possible that our failure in searching for a single aetiological factor will become more evident as details are elucidated; however, the disease is likely to continue to carry the “idiopathic” prefix for a long time.
P- Reviewer: Naito Y, Oda I, Tohda G S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58:642-646. [PubMed] |
| 2. | Cohen C. Granulomatous mastitis. A review of 5 cases. S Afr Med J. 1977;52:14-16. [PubMed] |
| 3. | Ozmen V, Cantürk Z, Celik V, Güler N, Kapkaç M, Koyuncu A, Müslümanoğlu M, Utkan Z. Breast Disease. Federation of Breast Diseases Society, Ankara: Güneş Medical Publishing 2012; 55-65. |
| 4. | Bakaris S, Yuksel M, Ciragil P, Guven MA, Ezberci F, Bulbuloglu E. Granulomatous mastitis including breast tuberculosis and idiopathic lobular granulomatous mastitis. Can J Surg. 2006;49:427-430. [PubMed] |
| 5. | Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Tuli R, O’Hara BJ, Hines J, Rosenberg AL. Idiopathic granulomatous mastitis masquerading as carcinoma of the breast: a case report and review of the literature. Int Semin Surg Oncol. 2007;4:21. [PubMed] |
| 7. | Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004;10:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Azlina AF, Ariza Z, Arni T, Hisham AN. Chronic granulomatous mastitis: diagnostic and therapeutic considerations. World J Surg. 2003;27:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Diesing D, Axt-Fliedner R, Hornung D, Weiss JM, Diedrich K, Friedrich M. Granulomatous mastitis. Arch Gynecol Obstet. 2004;269:233-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Erhan Y, Veral A, Kara E, Ozdemir N, Kapkac M, Ozdedeli E, Yilmaz R, Koyuncu A, Erhan Y, Ozbal O. A clinicopthologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast. 2000;9:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Tavassoli FA. Pathology of the Breast, 2nd ed. NewYork: McGraw-Hill 1999; 1099-1116. |
| 12. | Cserni G, Szajki K. Granulomatous Lobular Mastitis Following Drug-Induced Galactorrhea and Blunt Trauma. Breast J. 1999;5:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Pereira FA, Mudgil AV, Macias ES, Karsif K. Idiopathic granulomatous lobular mastitis. Int J Dermatol. 2012;51:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 14. | Gautier N, Lalonde L, Tran-Thanh D, El Khoury M, David J, Labelle M, Patocskai E, Trop I. Chronic granulomatous mastitis: Imaging, pathology and management. Eur J Radiol. 2013;82:e165-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Altınbas A, Ekiz F, Coban S, Yuksel O. Alfa 1 Antitripsin Eksikliği. Yeni Tıp Dergisi. 2012;29:138-141. |
| 16. | Schelfout K, Tjalma WA, Cooremans ID, Coeman DC, Colpaert CG, Buytaert PM. Observations of an idiopathic granulomatous mastitis. Eur J Obstet Gynecol Reprod Biol. 2001;97:260-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Oran EŞ, Gürdal SÖ, Yankol Y, Öznur M, Calay Z, Tunacı M, Soybir GR. Management of idiopathic granulomatous mastitis diagnosed by core biopsy: a retrospective multicenter study. Breast J. 2013;19:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012;15:119-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 19. | Al-Khaffaf B, Knox F, Bundred NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg. 2008;206:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg. 2007;31:1677-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Asoglu O, Ozmen V, Karanlik H, Tunaci M, Cabioglu N, Igci A, Selcuk UE, Kecer M. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Goldberg J, Baute L, Storey L, Park P. Granulomatous mastitis in pregnancy. Obstet Gynecol. 2000;96:813-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Sakurai K, Fujisaki S, Enomoto K, Amano S, Sugitani M. Evaluation of follow-up strategies for corticosteroid therapy of idiopathic granulomatous mastitis. Surg Today. 2011;41:333-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Gal-Gombos EC, Esserman LE, Odzer SL, Weisberg S, Wilson C, Poppiti RJ. Granulomatous mastitis: diagnosis by ultrasound-guided core biopsy. Breast J. 2001;7:129-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Han BK, Choe YH, Park JM, Moon WK, Ko YH, Yang JH, Nam SJ. Granulomatous mastitis: mammographic and sonographic appearances. AJR Am J Roentgenol. 1999;173:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Jorgensen MB, Nielsen DM. Diagnosis and treatment of granulomatous mastitis. Am J Med. 1992;93:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Salam IM, Alhomsi MF, Daniel MF, Sim AJ. Diagnosis and treatment of granulomatous mastitis. Br J Surg. 1995;82:214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Sato N, Yamashita H, Kozaki N, Watanabe Y, Ohtsuka T, Kuroki S, Nakafusa Y, Ota M, Chijiiwa K, Tanaka M. Granulomatous mastitis diagnosed and followed up by fine-needle aspiration cytology, and successfully treated by corticosteroid therapy: report of a case. Surg Today. 1996;26:730-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Rowe PH. Granulomatous mastitis associated with a pituitary prolactinoma. Br J Clin Pract. 1984;38:32-34. [PubMed] |
| 30. | Going JJ, Anderson TJ, Wilkinson S, Chetty U. Granulomatous lobular mastitis. J Clin Pathol. 1987;40:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Ozel L, Unal A, Unal E, Kara M, Erdoğdu E, Krand O, Güneş P, Karagül H, Demiral S, Titiz MI. Granulomatous mastitis: is it an autoimmune disease? Diagnostic and therapeutic dilemmas. Surg Today. 2012;42:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Coskun T, Kara E, Kaya Y, Guler Y, Kandiloglu AR, Goktan C. Granülomatöz Mastit: Cerrahi Tedavi-Rekuürrens İlişkisi. Meme Sağlığı Dergisi. 2006;2:26-30. |
| 33. | Bes C, Soy M, Vardi S, Sengul N, Yilmaz F. Erythema nodosum associated with granulomatous mastitis: report of two cases. Rheumatol Int. 2010;30:1523-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Salesi M, Karimifar M, Salimi F, Mahzouni P. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol Int. 2011;31:1093-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Taniguchi Y, Kagawa T, Ishibashi A, Horino T, Kumon Y, Terada Y. Weber-Christian disease associated with granulomatous mastitis: a variant type of Weber-Christian disease? Mod Rheumatol. 2011;21:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 36. | Letourneux C, Diemunsch P, Korganow AS, Akladios CY, Bellocq JP, Mathelin C. First report of granulomatous mastitis associated with Sjögren’s syndrome. World J Surg Oncol. 2013;11:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Altintoprak F, Karakece E, Kivilcim T, Dikicier E, Cakmak G, Celebi F, Ciftci IH. Idiopathic granulomatous mastitis: an autoimmune disease? ScientificWorldJournal. 2013;2013:148727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Thornton JW, Argenta LC, McClatchey KD, Marks MW. Studies on the endogenous flora of the human breast. Ann Plast Surg. 1988;20:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Taylor GB, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. 2003;35:109-119. [PubMed] |
| 40. | Paviour S, Musaad S, Roberts S, Taylor G, Taylor S, Shore K, Lang S, Holland D. Corynebacterium species isolated from patients with mastitis. Clin Infect Dis. 2002;35:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Ang LM, Brown H. Corynebacterium accolens isolated from breast abscess: possible association with granulomatous mastitis. J Clin Microbiol. 2007;45:1666-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Funke G, von Graevenitz A, Clarridge JE, Bernard KA. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. 1997;10:125-159. [PubMed] |
| 43. | Stary CM, Lee YS, Balfour J. Idiopathic granulomatous mastitis associated with corynebacterium sp. Infection. Hawaii Med J. 2011;70:99-101. [PubMed] |
| 44. | Mathelin C, Riegel P, Chenard MP, Tomasetto C, Brettes JP. Granulomatous mastitis and corynebacteria: clinical and pathologic correlations. Breast J. 2005;11:357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 45. | Ocal K, Dag A, Turkmenoglu O, Kara T, Seyit H, Konca K. Granulomatous mastitis: clinical, pathological features, and management. Breast J. 2010;16:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Akcan A, Akyildiz H, Deneme MA, Akgun H, Aritas Y. Granulomatous lobular mastitis: a complex diagnostic and therapeutic problem. World J Surg. 2006;30:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Al-Khawari HA, Al-Manfouhi HA, Madda JP, Kovacs A, Sheikh M, Roberts O. Radiologic features of granulomatous mastitis. Breast J. 2011;17:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Centers for Disease Control and Prevention (CDC). Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006-2008. MMWR Morb Mortal Wkly Rep. 2009;58:1317-1321. [PubMed] |
| 49. | Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 50. | Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis - report of 43 cases from iran; introducing a preliminary clinical practice guideline. Breast Care (Basel). 2013;8:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |