Published online Nov 16, 2014. doi: 10.12998/wjcc.v2.i11.717
Revised: July 30, 2014
Accepted: September 6, 2014
Published online: November 16, 2014
Processing time: 221 Days and 18 Hours
The management of patients with non-progressive metastatic colorectal cancer after six months of treatment has not yet been codified. The most relevant concerns are the effectiveness of maintenance vs discontinuation, and the tolerability of prolonged treatment. Here we report the case of a 72-year-old man affected by colorectal cancer with lung metastases who achieved a complete response after receiving capecitabine, oxaliplatin and bevacizumab for six months, and bevacizumab alone for six months. Bevacizumab was continued as maintenance regimen for more than three years. It was discontinued because of an arthroplasty. Fifty-eight months after beginning first-line treatment, the patient remains free from relapse. Adverse effects were minimal and easily controlled.
Core tip: A colorectal cancer patient with lung metastases received bevacizumab combined with chemotherapy for six months, and then bevacizumab monotherapy as maintenance treatment for more than three years. The patient achieved a complete response without evidence of side effects. He remains relapse free 58 mo after diagnosis.
- Citation: Stefano AD, Moretto R, Cella CA, Romano FJ, Raimondo L, Fiore G, Pietro FD, Pepe S, Placido SD, Carlomagno C. Bevacizumab maintenance in metastatic colorectal cancer: How long? World J Clin Cases 2014; 2(11): 717-723
- URL: https://www.wjgnet.com/2307-8960/full/v2/i11/717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i11.717
The management of metastatic colorectal cancer (mCRC) has evolved over the past decade[1]. Patients receiving irinotecan[2], oxaliplatin[3] and fluorouracil achieve the best outcome (median survival, approximately 21 mo), regardless of treatment sequence[4]. Adding biological drugs such as anti-vascular endothelial growth factor (VEGF) or anti-epidermal growth factor receptor (EGFR) monoclonal antibodies to chemotherapy improved survival further[5]. The anti-VEGF monoclonal antibody bevacizumab significantly prolonged survival when added to first-line[6] or second-line chemotherapy[7]. Recent clinical trials showed a clinical benefit in patients receiving bevacizumab even beyond progression[8,9].
Although often used in clinical practice, maintenance therapy in non-progressive patients after four-six months of chemotherapy ± biological drugs is not yet codified in terms of duration (indefinitely until disease progression?) and type of drugs (same chemotherapy regimen, simplified chemotherapy, targeted drug alone?). The most relevant concerns are the effectiveness of maintenance vs discontinuation, and the tolerability of such prolonged treatment.
Here, we report the case of a 72-year-old man affected by mCRC who obtained a complete response after treatment with capecitabine, oxaliplatin and bevacizumab for six months, followed by bevacizumab alone for a further six months. He then continued with bevacizumab as maintenance treatment for the following three years with no evidence of disease relapse or toxicity.
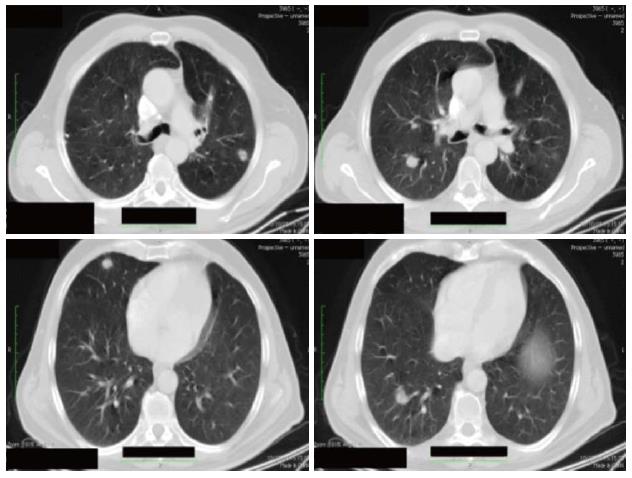
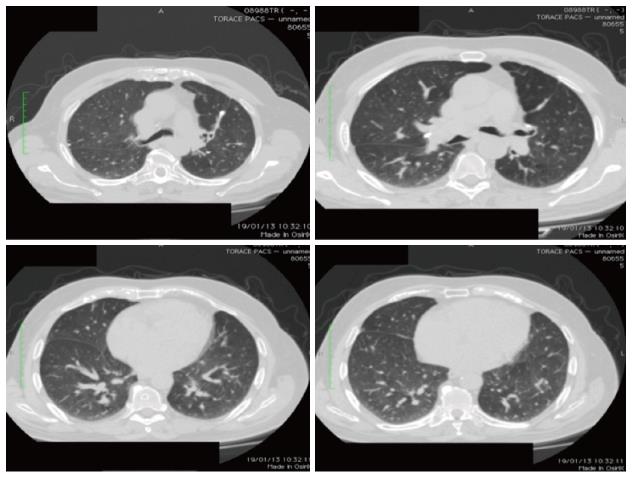
In October 2008, a 72-year-old man underwent left hemicolectomy for an obstructing mass located at the sigmoid-rectal junction. The pathological diagnosis was an undifferentiated (G3) adenocarcinoma, invading through the muscularis mucosae up to the peri-colic fat tissue (pT3); 12 nodes were isolated, all of which were metastatic (pN2). Radiological staging by contrast enhanced CT-scan showed several lung lesions (4 measurable) up to 20 mm in diameter (Figure 1). No liver metastases were detected. KRAS mutational status showed a mutation in codon 12 of exon 2.
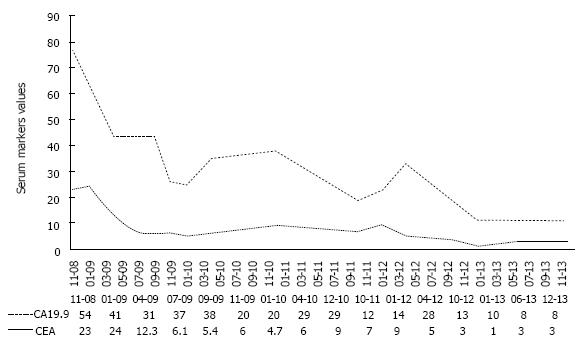
The patient was referred to the Division of Medical Oncology of the University of Naples “Federico II” in November 2008 to begin first-line chemotherapy. He was in good condition (ECOG performance status = 1), and was only taking an ACE-inhibitor for arterial hypertension. Serum tumor markers exceeded the upper normal level: CA19.9 was 54 U/L (normal values < 37 U/L), and CEA 23.3 ng/mL (normal values < 5 ng/mL).
He began chemotherapy with the XELOX + bevacizumab schedule (capecitabine 1000 mg/m2 twice daily, days 1-14; oxaliplatin 130 mg/m2, and bevacizumab 7.5 mg/kg day 1, every 21 d). The work-up consisted of blood cell count and hematology before each cycle, and CT-scan every three months (or four cycles). Arterial blood pressure was measured before and after bevacizumab administration on day 1 and, thereafter, once daily with an electronic sphygmomanometer. The worse adverse events were: grade 1 peripheral neuropathy, grade 2 arterial hypertension, grade 1 hand-foot syndrome and grade 2 thrombocytopenia. Due to the worsening of arterial hypertension after the first treatment cycle, a diuretic and a calcium channel blocker were added to the anti-hypertensive treatment, which resulted in good blood pressure control. Bevacizumab administration remained unchanged, and blood pressure was well controlled.
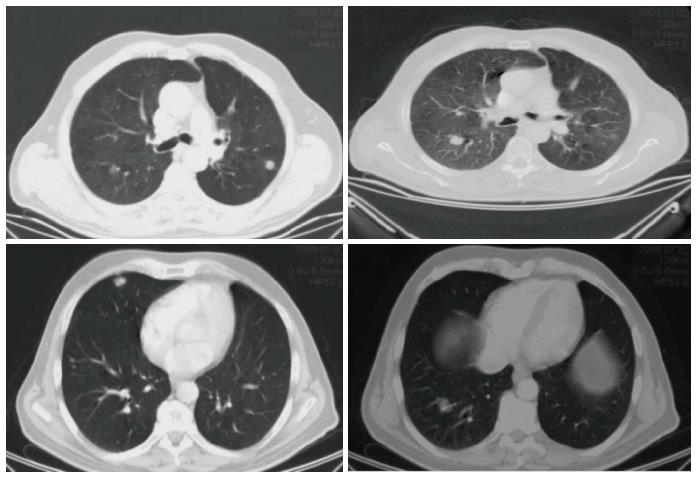
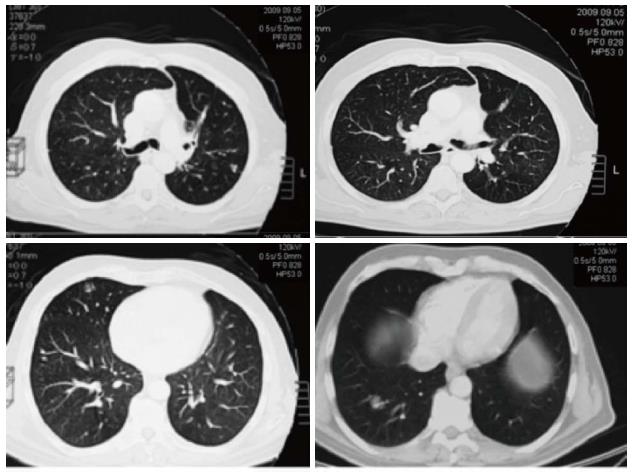
Re-staging performed by whole-body CT scan after the fourth and the eighth (Figure 2) cycle of treatment showed a stable disease according to RECIST criteria. Thus, after completing eight courses (six months), capecitabine and oxaliplatin were discontinued and maintenance with bevacizumab alone (7.5 mg/kg every 21 d) was started. During the maintenance period, re-staging by whole-body CT scan was scheduled every three months. In September 2009, the CT scan showed a significant reduction of the maximum diameter of the four measurable lung metastases (Figure 3), which were no longer detected after a further 3 mo of treatment (November 2009) (Figure 4).
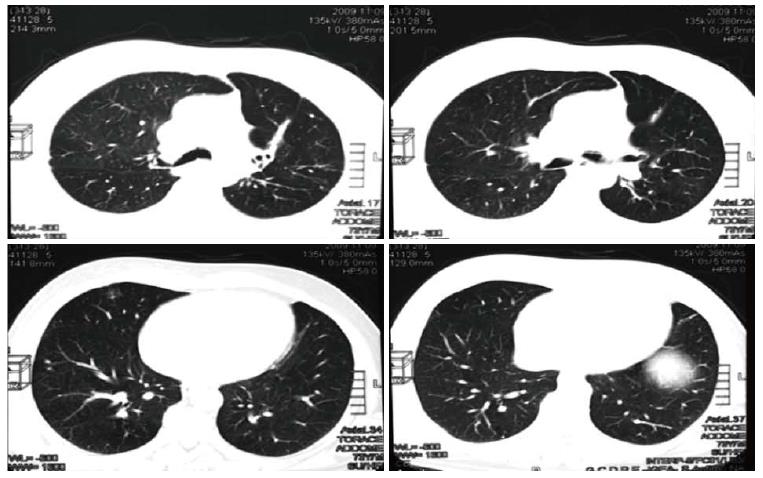
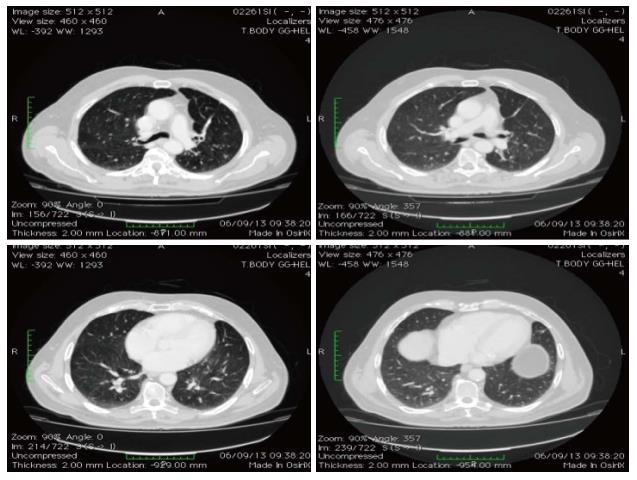
The patient continued with bevacizumab for the following 44 mo, remaining free from metastases (Figure 5), and without reporting any adverse event. Serum marker levels mirrored the outcome of the lung metastases throughout treatment and follow-up (Figure 6). In January 2013, bevacizumab was discontinued to enable the patient to undergo hip arthoplasty because of right coxarthrosis. Currently (June 2014), 68 mo after diagnosis, the patient has a good ECOG performance status with no evidence of metastatic disease (Figure 7). He attends follow-up controls every three months.
Here we report the case of a 72-year-old man with multiple lung metastases from a colon adenocarcinoma who achieved a complete radiological response after 12 mo of treatment: six with chemotherapy plus bevacizumab and six with bevacizumab alone. Bevacizumab alone was continued as maintenance therapy with bevacizumab alone for 44 mo, and patient experienced only a few side effects that were easily managed, and he enjoyed a good quality of life throughout the entire course of treatment.
Bevacizumab in combination with fluoropyrimidine-based chemotherapy is indicated for the treatment of patients with metastatic carcinoma of the colon or rectum, and it is recommended that treatment be continued until disease progression or unacceptable toxicity. However, when used as monotherapy, bevacizumab is not known to induce tumor regression or disappearance[7].
Saltz et al[10] reported that the addition of bevacizumab to an oxaliplatin-based regimen in the first-line setting significantly prolonged median progression-free survival (9.4 mo vs 8.0 mo; P = 0.0023), but did not significantly improve overall survival or the response rate[10]. However, many patients discontinued bevacizumab combined with chemotherapy for reasons other than progression or toxicity. A pre-defined analysis of on-treatment patients showed a much higher magnitude of benefit in progression-free survival for treatment until progression (HR = 0.63) than pre-progression discontinuation (HR = 0.83), which suggests that it is important to continue bevacizumab to maximize the benefit of its addition to chemotherapy[10].
The role of maintenance therapy in mCRC is still controversial. The most recent ESMO consensus guidelines suggest that treatment discontinuation or maintenance are feasible options after 4-6 mo of full-dose first-line therapy[11]. Two randomized trials-MACRO[12], and DREAM[13]-support the role of maintenance with bevacizumab alone[12] or with erlotinib[13].
However, the magnitude of the benefit of maintenance therapy over the discontinuation approach has been addressed in 3 clinical trials, comparing maintenance with complete treatment discontinuation. The CAIRO 3 trial after a 4 mo’ period of treatment (CAP-OX plus bevacizumab regimen) randomized not progressive patients to maintenance with capecitabine plus bevacizumab or no further therapy. First and second progression-free survival times and time to second progression were improved in the maintenance arm.
The SAKK (41/06) trial was designed to demonstrate the non-inferiority of complete discontinuation of treatment as compared to maintenance with bevacizumab a after 4-6 mo of chemotherapy plus bevacizumab. The non-inferiority was not statistically demonstrated, suggesting that maintenance with bevacizumab might be considered an appropriate option.
At ASCO 2014 the results of AIO0207 were presented. After 24 wk of induction therapy with fluoropyrimidines, oxaliplatin and bevacizumab, maintenance with fluoropyrimidines plus bevacizumab was compared with bevacizumab alone or with complete discontinuation. Maintenance treatment with fluoropyrimidines and bevacizumab prolongs progression free survival and time to failure of strategy with respect to complete discontinuation.
When we decided the maintenance strategy for our patient, there was no information available about the role of fluoropyrimidines in this condition, therefore only bevacizumab was continued. Of note, our patient achieved a complete response after 12 mo of treatment and a very long progression-free survival, which supports continuing bevacizumab after stopping chemotherapy.
Another issue we were able to address is the tolerability of very long treatment with bevacizumab. Our patient was 72-year-old when metastatic disease was diagnosed, and was affected by arterial hypertension. Bleeding, hypertension and venous thromboembolic events are the most frequent side effects of bevacizumab recorded, also in a randomized clinical study[14]. Although hypertension was much more frequent in patients treated with bevacizumab than in controls, only arterial thromboembolic events were significantly higher in the bevacizumab-treated patients aged ≥ 70 years (6.7% vs 3.2% in the control group)[14]. It is noteworthy that patients enrolled in clinical trials are selected for their very good conditions, and with very limited comorbidities. However, similar results were in that BRITE observational study, where only a modest increase of arterial thromboembolic events was observed in elderly patients: unadjusted rate per 100 patients-years was 1.4 for patients < 75 years old, 4.0 for patients aged 75-80, and 4.8 for older patients)[15]. Moreover, in the BRITE study, the median time to occurrence of bleeding and arterial thromboembolic events was about 5 mo and the risk of cardiovascular side effects did not increase with the duration of bevacizumab treatment[15].
The AVEX trial[16] was the first phase III trial to prospectively evaluate the use of bevacizumab in elderly patients (≥ 70-year-old) affected by mCRC. Bleeding (25.4%), arterial hypertension (19.4%), venous thromboembolic (11.9%) and arterial thromboembolic events (4.5%) were the most common all-grade adverse events in the cohort of 140 patients receiving bevacizumab[16].
In our patient, arterial hypertension worsened after the first administration of bevacizumab, and was successfully managed with anti-hypertensive therapy, without discontinuing bevacizumab.
In conclusion, our report, although based on a single patient, supports the feasibility and, possibly, the benefit of continuing bevacizumab as maintenance treatment for a very long period. Moreover, the case reported here suggests that this regimen can be administered even in elderly patients provided they are fit. Indeed, they can have the same benefits as adult subjects, possibly without a significant increase in toxicity.
We thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for editing the text.
The case reports the clinical case of a colorectal cancer patient presenting with lung metastases.
He underwent first line chemotherapy with capecitabine, oxaliplatin and bevacizumab as induction treatment for six months, after which he continued receiving bevacizumab monotherapy for more than three years.
He achieved a complete response and is currently free from relapse.
The long progression-free survival time and the absence of side effects indicates that bevacizumab-based maintenance treatment is beneficial in mCRC patients. However, although some studies reported a benefit of maintenance therapy in PFS or TTF, the definite role of maintenance on survival and the best maintenance regimen might be further investigated in properly designed clinical trials.
It is a very interested topic for physicians of multiple specialties such as Internal Medicine, Oncology, Surgery, Family Medicine. The manuscript is very well written.
P- Reviewer: Casadesus D, Herszenyi L, Lu F, Nishida T S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2273] [Cited by in RCA: 2222] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 3. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [PubMed] |
| 4. | Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 794] [Article Influence: 37.8] [Reference Citation Analysis (1)] |
| 5. | Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 6. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7734] [Article Influence: 368.3] [Reference Citation Analysis (1)] |
| 7. | Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1726] [Article Influence: 95.9] [Reference Citation Analysis (1)] |
| 8. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 897] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 9. | Masi G, Loupakis F, Salvatore L, Cremolini C, Fornaro L, Schirripa M, Fea E, Granetto C, Antonuzzo L, Giommoni E. A randomized phase III study evaluating the continuation of bevacizumab (bv) beyond progression in metastatic colorectal cancer (mcrc) patients (pts) who received bv as part of first-line treatment: results of the BEBYP trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. 2012;23:9. |
| 10. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2271] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 11. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J. ESMO Consensus Guidelines for management of patients with colon and rectal cancer: A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 12. | Díaz-Rubio E, Gómez-España A, Massutí B, Sastre J, Abad A, Valladares M, Rivera F, Safont MJ, Martínez de Prado P, Gallén M. First-line XELOX plus bevacizumab followed by XELOX plus bevacizumab or single-agent bevacizumab as maintenance therapy in patients with metastatic colorectal cancer: the phase III MACRO TTD study. Oncologist. 2012;17:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Tournigand C, Samson B, Scheithauer W, Lledo G, Viret F, Andre T, Ramée JF, Tubiana-Mathieu N, Dauba J, Dupuis O. Bevacizumab (Bev) with or without erlotinib as maintenance therapy, following induction first-line chemotherapy plus Bev, in patients (pts) with metastatic colorectal cancer (mCRC): Efficacy and safety results of the International GERCOR DREAM phase III trial. J Clin Oncol. 2012;30 suppl:3500. |
| 14. | Cassidy J, Saltz LB, Giantonio BJ, Kabbinavar FF, Hurwitz HI, Rohr UP. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Kozloff MF, Berlin J, Flynn PJ, Kabbinavar F, Ashby M, Dong W, Sing AP, Grothey A. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology. 2010;78:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Cunningham D, Lang I, Lorusso V, Ocvirk J, Shin D, Jonker D, Osborne S, Andre N, Waterkamp D, Saunders M. Bevacizumab (bev) in combination with capecitabine (cape) for the first-line treatment of elderly patients with metastatic colorectal cancer (mCRC): Results of a randomized international phase III trial (AVEX). J clin Oncol. 2013;30 suppl:3502. |