Published online Nov 16, 2014. doi: 10.12998/wjcc.v2.i11.614
Revised: July 11, 2014
Accepted: September 17, 2014
Published online: November 16, 2014
Processing time: 180 Days and 9.8 Hours
Acute ischemic stroke (AIS) is a common medical problem associated with significant morbidity and mortality worldwide. A small proportion of AIS patients meet eligibility criteria for intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator, and its efficacy for large vessel occlusion is poor. Therefore, an increasing number of patients with AIS are being treated with endovascular mechanical thrombectomy when IVT is ineffective or contraindicated. Rapid advancement in catheter-based and endovascular device technology has led to significant improvements in rates of cerebral reperfusion with these devices. Stentrievers and modern aspiration catheters have now surpassed earlier generation devices in the degree and rapidity of revascularization. This progress has been achieved with no concurrent increase in risk of major complications or mortality, both when used alone or in combination with IVT. The initial randomized controlled trials comparing endovascular therapy to IVT for AIS failed to show superior outcomes with endovascular treatment, but key limitations of each trial may limit the significance of these results to current practice. While endovascular devices and operator experience continue to evolve, we are optimistic that this will be accompanied by improvements in patient outcomes. This review highlights the major endovascular devices used in current practice and the trials which have investigated their efficacy.
Core tip: This review discusses the critical advancements in endovascular device technology for the treatment of acute ischemic stroke. Endovascular mechanical thrombectomy is becoming an increasingly utilized treatment approach for patients in whom intravenous thrombolysis with recombinant tissue plasminogen activator is ineffective or contraindicated. While three recent randomized controlled trials found no benefit of endovascular thrombectomy over intravenous therapy, it is important for clinicians to understand the limitations of these trials and recognize the expected key role of endovascular therapy in the future management of stroke patients.
- Citation: Przybylowski CJ, Ding D, Starke RM, Durst CR, Crowley RW, Liu KC. Evolution of endovascular mechanical thrombectomy for acute ischemic stroke. World J Clin Cases 2014; 2(11): 614-622
- URL: https://www.wjgnet.com/2307-8960/full/v2/i11/614.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i11.614
The annual incidence of stroke in the United States is approximately 795000[1]. Stroke is the second leading cause of lost disability-adjusted life-years in high-income countries and the second most common cause of mortality worldwide[2,3]. Approximately 80% of strokes are ischemic and 20% are hemorrhagic[4]. Acute ischemic stroke (AIS) is caused by a focal interruption of cerebral blood flow, most commonly due to occlusion of a major cerebral artery by local thrombosis or embolus. The resulting ischemia leads to tissue damage through a complex pathophysiological response of excitotoxicity, peri-infarction depolarization, inflammation and apoptosis[5].
The goal of therapy for AIS is to achieve cerebral reperfusion before neurological damage becomes irreversible. The correlation between vessel recanalization and favorable neurological outcome is well-studied[6,7], although additional factors such as stroke severity and age are also likely to have a significant impact on clinical outcome[8,9]. Currently, the only FDA-approved treatment with level 1 evidence for AIS is intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (alteplase) within three hours of symptom onset[10]. Additional trials have demonstrated that extending this time window to 4.5 h is beneficial in appropriately selected patients[11,12]. However, few patients (< 10%) meet eligibility criteria for this therapy[13-15]. Additionally, larger and more proximally-located thrombi may be relatively resistant to IVT[16-21]. Successful recanalization of large vessel occlusion (LVO) with IVT alone is infrequent, ranging from 10% in internal carotid artery (ICA) occlusions to 30% in middle cerebral artery occlusions, and IVT is associated with a risk of systemic and intracerebral hemorrhage (ICH)[22].
These limitations have led to the exploration of alternative or complementary treatment approaches for AIS. Endovascular mechanical thrombectomy has developed over the past decade as a safe and effective intervention. Rapid advancement in catheter-based and endovascular device technology has led to an increasing number of patients with AIS being treated when IVT is ineffective or contraindicated[14]. Here, we review the evolution of endovascular mechanical thrombectomy devices for the treatment of AIS.
Endovascular treatment of AIS began with intra-arterial (IA) infusion of thrombolytic agents. Several studies investigating these therapies reported favorable rates of vessel recanalization and neurological outcomes[23-25]. Given the relatively favorable risk to benefit profiles of current mechanical thrombectomy devices, IA thrombolysis is infrequently used in modern endovascular AIS therapy. This was followed by the implementation of balloon angioplasty and microwire techniques to mechanically disrupt thromboemboli[26,27]. Additionally, intracranial stents were shown to be effective at restoring blood flow when deployed within an occluded vessel[28,29].
Endovascular retrieval devices were first developed to recover errant coils and other foreign bodies that had embolized within the cerebral circulation during endovascular procedures[30-32]. The development of devices to remove occlusive thromboemboli was thus a natural extension of pre-existing technology. Endovascular mechanical thrombectomy involves physical extraction of the thrombus through a catheter. Due to the anatomical limitations imposed by vascular anatomy on currently available thrombectomy catheters, thrombi in large ICA, the Circle of Willis and the first two branches of the anterior (A1 and A2), middle (M1 and M2) and posterior (P1 and P2) cerebral arteries) are the most readily accessible. Smaller branches of the cerebral circulation are often too narrow and tortuous to undergo successful mechanical thrombectomy.
Alternative treatment methods for strokes from LVO are an important development, as medical management is often unsuccessful, and these strokes are associated with high rates of morbidity and mortality[25]. The two main methods of endovascular mechanical thrombectomy for LVO include: (1) physical grasping and removal of thrombi with retrieval devices and (2) aspiration of occlusive thrombi with suction devices (Table 1).
| Device | Manufacturer | Mechanism |
| Merci retriever | Concentric Medical | Thrombus retrieved with helical snare |
| Penumbra system | Penumbra Inc. | Thromboaspiration |
| Solitaire FR | eV3 Endovascular | Thrombus incorporated into struts of deployed stent |
| Trevo Pro | Stryker neurovascular | Thrombus incorporated into struts of deployed stent |
The Merci Retriever (Concentric Medical, Mountainview, CA) was FDA-approved in August 2004 as the first clot retriever device in the United States. This device utilizes memory shaped nitinol (nickel titanium) material to convert from a straight to helical configuration to grasp the thrombus. In this procedure[33]: (1) the retriever is advanced through the thrombus in its straight configuration; (2) two to three helical loops are deployed beyond the thrombus; (3) the device is retracted to contact the thrombus, and proximal loops are deployed within the thrombus; (4) a balloon guide catheter located in the common or internal carotid artery is inflated to control intracranial blood flow; and (5) three to five clockwise rotations are performed to fully ensnare the thrombus, and the Merci Retriever-thrombus complex and microcatheter are removed together.
The Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial was a prospective, non-randomized, multicenter trial that first evaluated this device[34]. Recanalization, defined as Thrombolysis in Myocardial Infarction (TIMI) grade 2 or 3 flow in all treatable vessels, was achieved in 48% (68/141) of patients. Patients with recanalization had better neurological outcomes (P < 0.0001), as determined by modified Rankin Score (mRS) of 2 or less at 90 d, and lower mortality rates (P = 0.01) than those without recanalization, and procedure-related complication and symptomatic ICH rates were comparable to trials of IV t-PA, combined IV/intra-arterial t-PA, and intra-arterial prourokinase[10,25,35].
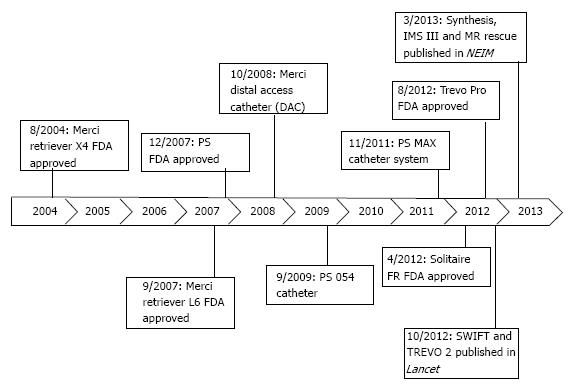
Newer device generations (Figure 1) have moderately improved rates of recanalization[36]. One such advancement was the Distal Access Catheter (DAC; Concentric Medical) in 2008. The DAC has a flexible distal shaft that facilitates its navigation around the anterior genu of the ICA, beyond the origin of the ophthalmic artery[37]. This improved the navigation of the Merci Retriever through the carotid siphon with each pass, improving procedural efficiency.
The Penumbra System (PS) (Penumbra Inc., Alameda, CA) was FDA-approved in December 2007 and utilizes aspiration for thrombus extraction. In this procedure[38]: (1) the PS catheter is advanced through a guide catheter to a point just proximal to the occlusion; (2) a microwire called a separator is repeatedly passed through the thrombus in order to fragment the clot; and (3) constant suction is applied to the PS catheter to aspirate the thrombus fragments.
The PS was evaluated in a prospective, multicenter study of 125 patients with National Institute of Health Stroke Scale (NIHSS) score ≥ 8 who were ineligible for or refractory to IVT[38]. Recanalization (TIMI ≥ 2) was achieved in a high proportion (81.6%) of patients without significantly different complication rates than those seen in the MERCI trials. Good clinical outcome (mRS ≤ 2) was observed in 25% of patients at 30-d follow-up. More recent generations of the PS include the 054 Reperfusion catheter (2009) and the MAX Reperfusion catheter line (2011). These devices achieve greater aspiration force due to larger proximal lumens[39]. The 054 Reperfusion device was found to accomplish recanalization at a median time of 20 min[40], significantly less than the median time of 45 min reported in the penumbra pivotal trial using previous generation technology.
Stentrievers utilize a retrievable stent to engage and remove the thrombus. In this procedure[41]: (1) the stentriever is advanced within a microcatheter through the thrombus until it is a few millimeters distal to the clot; (2) the stent is deployed, incorporating the thrombus into the stent struts and displacing it radially to the vascular wall; and (3) after three to five minutes, the microcatheter and stentriever are removed together under continuous proximal aspiration with a syringe. This must be performed cautiously, as at least one case of intracranial extravasation during device withdrawal has been reported[42]. It is also possible to perform a control angiogram while the stentriever is deployed, which can confirm flow restoration. However, since this may promote distal migration of thrombus fragments, the utility of performing an angiogram during stentriever deployment is controversial.
The Solitaire FR (eV3 Endovascular, Irvine, CA) was approved by the FDA in March 2012. Initial non-randomized case series with the Solitaire FR demonstrated high rates of recanalization (89%-96%) and improved rates of favorable clinical outcome (mRS ≤ 2; 42%-69%) compared to earlier devices[29,41,43-45]. The Solitaire FR was then directly compared to the Merci Retriever in the SOLITAIRE™ with the intention for thrombectomy (SWIFT) trial (Table 2)[46]. This was a parallel-group, non-inferiority trial of 113 patients randomized to either the Solitaire (n = 58) or Merci (n = 55) device. The primary outcome (TIMI ≥ 2) was more likely to be achieved in the Solitaire group than the Merci group (64% vs 24%; P < 0.0001 non-inferiority; P = 0.0001 superiority). Additionally, patients in the Solitaire group were more likely to achieve a good neurological outcome (mRS ≤ 2) at 90 d (58% vs 33%; P < 0.0001 non-inferiority; P = 0.02 superiority) and had a lower 90-d mortality rate (17% vs 38%; P = 0.0001 non-inferiority; P = 0.02 superiority) than those in the Merci group. Subsequent prospective and retrospective studies have continued to demonstrate high rates of vessel recanalization and good clinical outcomes with the Solitaire FR[47,48].
| Trial | Treatment arms | n | Revascularizationa (%) | Good outcomebc (%) | Symptomatic ICH (%) | Mortalityb (%) |
| SWIFT | Merci Retriever | 55 | 67 | 33 | 11 | 38 |
| Solitaire FR | 58 | 89 | 58 | 2 | 17 | |
| TREVO 2 | Merci Retriever | 90 | 60 | 22 | 2 | 24 |
| Trevo Pro | 88 | 86 | 40 | 4 | 33 | |
| SYNTHESISd | IVT | 181 | NR | 35 | 6 | 6 |
| EVT | 181 | NR | 30 | 6 | 8 | |
| IMS III | IVT | 222 | NR | 39 | 6 | 22 |
| IVT + EVT | 434 | e | 41 | 6 | 19 | |
| MR Rescue | Penumbral, IVT | 34 | 93 | 26 | 6 | 21 |
| Penumbral, EVT | 34 | 67 | 21 | 9 | 18 | |
| Nonpenumbral, IVT | 20 | 78 | 10 | 0 | 30 | |
| Nonpenumbral, EVT | 30 | 77 | 17 | 0 | 20 |
The Trevo Pro (Stryker Neurovascular, Kalamazoo, MI) is another retrievable stent system which was approved by the FDA in August 2012. Similar to the Solitaire FR, the Trevo Pro was found to be superior to the Merci Retriever in a head-to-head randomized study[49]. The Thrombectomy REvascularization of Large Vessel Occlusions in Acute Ischemic Stroke trial assigned patients with AIS from LVO to either the Trevo Pro (n = 88) or Merci Retriever (n = 90) device. Patients in the Trevo Pro group were significantly more likely to reach the primary outcome, defined as Thrombolysis in Cerebral Ischemia (TICI) grade ≥ 2, (86% vs 60%; P < 0.0001 superiority) and achieve a good 90-d neurological outcome (mRS ≤ 2; P = 0.013) than those in the Merci Retriever group. No significant difference was observed in the safety profile (a composite of symptomatic ICH and procedure-related complications; P = 0.1826) or 90-d mortality rates (P = 0.1845) of these two devices.
A review of 13 prospective trials endorsed improved rates of vessel recanalization with the newer generation stentriever devices[50]. While early trials (mainly utilizing IA thrombolysis and the Merci Retriever) reported recanalization rates of approximately 50%, recent trials with stentrievers consistently reported rates of approximately 85%. A significantly greater time from symptom onset to endovascular treatment in more recent trials was also observed. This may explain their finding that although vessel recanalization rates have significantly improved over time, functional outcomes remain relatively stagnant. Nevertheless, stentrievers and large bore aspiration catheters have become the dominant endovascular devices used to treat AIS in modern practice. A recent prospective trial found no major differences in the efficacy or safety of the Solitaire FR and Trevo Pro[51].
The MAX reperfusion catheters allowed for the development of direct aspiration as an additional thrombectomy technique. Direct suction can be applied from the PS device or a syringe plunger connected to the proximal hub of the catheter. Previously, this technique was limited by the challenges of tracking an aspiration catheter through the intracranial circulation, but the improved trackability of the MAX reperfusion catheters has facilitated its development[37]. Furthermore, these catheters can still be used in combination with other endovascular devices. The ADAPT technique[52] is an increasingly utilized approach which combines modern aspiration and retrieval technology. Direct aspiration with a large bore aspiration catheter (commonly MAX reperfusion system) is first performed. If direct aspiration fails, stentrievers, balloons and stents can be still be passed through the catheter. A recent retrospective series of 98 patients by Turk et al[53] reported revascularization (TICI ≥ 2b) in 78% of cases following direct aspiration. When stentrievers were used following failed direct aspiration, this rate rose to 95%, a previously unparalleled result.
The Penumbra 3D Separator is the newest generation PS device currently being investigated in randomized controlled trials. It is designed to combine stentriever and direct aspiration technology into a single device. The new separator device is configured similarly to a stentriever, with an additional radial dimension to fragment the clot under continuous direct aspiration. The stent struts are designed to minimize vessel contact and thus theoretically reduce iatrogenic injury to the endothelium. An initial prospective study of 20 patients treated with the Penumbra 3D Separator demonstrated vessel recanalization (TICI ≥ 2b) and favorable neurological outcome (mRS ≤ 2) in 85% and 50% of patients, respectively[54].
Due to the promising results from early pilot trials of endovascular mechanical thrombectomy for AIS[55,56], randomized controlled trials were undertaken to evaluate the benefit of endovascular therapy compared to IVT in a more rigorous fashion.
The Interventional Management of Stroke (IMS) III trial[57] randomly assigned 656 patients who had received IVT within three hours of AIS symptom onset to receive additional endovascular therapy (n = 434) or IVT alone (n = 222) in a 2:1 ratio. In the endovascular group, 330 patients received treatment: IA thrombolysis alone (n = 160), mechanical thrombectomy alone (n = 57), IA thrombolysis plus mechanical thrombectomy (n = 97) and combinations of multiple mechanical thrombectomy devices with or without IA thrombolysis (n = 16). There was no significant difference between the endovascular and IVT groups for achieving a 90-d mRS ≤ 2 (40.8% and 38.7%, respectively; 95%CI: -6.1 to 9.1). Additional subgroup analyses showed no difference between the two groups in patients with NIHSS ≥ 20 (95%CI: -4.4 to 18.1) or NIHSS < 20 (95%CI, -10.8 to 8.8). Similar rates of symptomatic ICH (6.2% in the endovascular group and 5.9% in the IVT group; P = 0.83) and 90-d mortality (19.1% in the endovascular group and 21.6% in the IVT group; P = 0.52) were observed.
Enrollment and treatment of patients in the endovascular arm of this trial was not optimal. Over 20% of patients in the endovascular arm were included for analysis despite not receiving any endovascular therapy (due to lack of LVO on angiography). Notably, subgroup analysis of patients with LVO confirmed by CTA showed that endovascular therapy was associated with better functional outcomes than IVT alone (P = 0.01)[58]. The time to endovascular treatment was also significantly longer in the IMS III trial compared to the previous IMS I and II trials. These earlier trials demonstrated that there is a close association between time to reperfusion and neurological outcome, with a linear decrease in probability of good neurological outcome with time[59]. Thus, this treatment delay may have reduced the clinical benefit of endovascular therapy in this trial. Lastly, of those treated, less than 5% were treated with stentrievers, either alone or in combination with other devices. This likely contributed to only 40% of patients achieving TICI grade 2b or 3 vessel recanalization[60].
The SYNTHESIS Expansion trial[61] randomly assigned 362 patients with AIS within 4.5 h of symptom onset to either endovascular therapy (n = 181) or IVT (n = 181). Patients in the endovascular group who underwent treatment (n = 165) received either IA thrombolysis (n = 109) alone or in combination with mechanical thrombectomy (n = 56) without any prior IVT. Survival-free disability (mRS ≤ 1) at 90 d, adjusted for key variables (age, sex, initial NIHSS grade and history of atrial fibrillation) did not significantly differ between the endovascular and IVT groups (30.4% and 34.8%, respectively; P = 0.16). Secondary outcomes including NIHSS score ≤ 6, neurological deterioration, mortality, symptomatic ICH, and recurrent AIS also did not significantly differ between groups.
Again, the protocol of this trial likely resulted in enrollment of patients who were not suitable candidates for endovascular therapy under current recommendations. No preoperative imaging was required to confirm LVO prior to randomization, and a significant portion of patients (> 33%) had a NIHSS ≤ 10. Additionally, the majority of patients in the endovascular arm received interventions that would no longer be considered standard of care. Only 13% of patients in the endovascular arm were treated with stentrievers[62], while 60% were treated with IA thrombolysis alone without mechanical retrieval. Because vessel recanalization rates were not reported, it is unclear if these patients received optimal therapeutic effect.
The MR Rescue trial[63] randomized 118 patients with large vessel anterior circulation strokes to either mechanical thrombectomy (n = 64) or IVT (n = 54) within eight hours of symptom onset. Patient groups were also stratified based on pre-treatment imaging into favorable or non- penumbral patterns. Some studies have suggested that measuring the extent of salvageable brain tissue or ischemic penumbra on preoperative imaging may identify patients who could preferentially benefit from endovascular therapy[64-67]. Favorable penumbral pattern was defined as a predicted infarct core of 90 mL or less and a proportion of infarct tissue within the at-risk region of 70 mL or less after pre-treatment magnetic resonance imaging or computed tomography. Results showed no significant difference in mean 90-d mRS observed among groups, both in the overall cohort (P = 0.99) or when stratified based on penumbral pattern (favorable, P = 0.23; non-penumbral, P = 0.32). No differences in the rates of symptomatic ICH (P = 0.24) or mortality (P = 0.75) were observed between groups. These results correlate with findings from a recent study which showed that a non-perfect preoperative ASPECT score did not significantly affect functional outcome[68].
No patients in the endovascular arm of the MR Rescue trial were treated with stentrievers. Similar to IMS III, this likely contributed to the low overall rate of recanalization. Only 27% of patients in the endovascular arm achieved recanalization of TICI grade 2b or 3[60]. Additionally, this trial may have been underpowered due to the relatively low number of patients in each group.
While these trials provided valuable preliminary data for the assessment of endovascular intervention for AIS, each had significant limitations[60,62]. In SYNTHESIS and IMS III, patients with LVO were not appropriately selected based on preoperative imaging. In all three trials, due to the pace of advancement in endovascular technologies, a minority of patients were treated with the most modern endovascular devices. Stentrievers were used infrequently in all three studies, which resulted in vessel recanalization rates below current standards. There is evidence that recanalization is associated with improved functional outcomes and reduced mortality[7]. Thus, the generalizability of the results from these trials to modern endovascular stroke practice is limited, and future randomized controlled trials are still needed.
As supported by the subgroup analysis of patients with CTA-positive LVO from the IMS III trial, evidence still supports the use of endovascular mechanical thrombectomy for LVO within eight hours of symptom onset. Importantly, none of these trials raised questions about the safety of endovascular therapy. Recanalization is now possible in over 80% of cases, and for many patients, endovascular therapy is the only available treatment option. As endovascular devices and operator experience continue to evolve, improvements in patient outcomes are expected. Future trials will need to focus on proper patient selection and achieving optimal therapeutic effect (vessel recanalization) with modern endovascular devices[69]. Three ongoing clinical trials (THERAPY, SWIFT-PRIME, and POSITIVE) appear to have incorporated these key principles into their study design.
Endovascular mechanical thrombectomy involves the physical extraction of an occluding thromboembolus via grasping devices and/or direct/indirect aspiration. Over the past decade, advancements in catheter-based and endovascular device technology have led to strong improvements in rates of vessel recanalization. Initial randomized trials failed to show benefit of endovascular therapy over IVT, but limitations in study design have abated widespread acceptance of their conclusions. Future randomized trials evaluating endovascular mechanical thrombectomy for AIS will need to enroll and treat patients based off of the currently accepted standards of care.
P- Reviewer: Soria F, Sobrino T S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 971] [Cited by in RCA: 956] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 2. | Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3532] [Cited by in RCA: 3496] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 3. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9576] [Article Influence: 736.6] [Reference Citation Analysis (0)] |
| 4. | Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1239] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 5. | Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2802] [Cited by in RCA: 2837] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 6. | Kharitonova TV, Melo TP, Andersen G, Egido JA, Castillo J, Wahlgren N. Importance of cerebral artery recanalization in patients with stroke with and without neurological improvement after intravenous thrombolysis. Stroke. 2013;44:2513-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1126] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 8. | Almekhlafi MA, Davalos A, Bonafe A, Chapot R, Gralla J, Pereira VM, Goyal M. Impact of Age and Baseline NIHSS Scores on Clinical Outcomes in the Mechanical Thrombectomy Using Solitaire FR in Acute Ischemic Stroke Study. AJNR Am J Neuroradiol. 2014;35:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Chalouhi N, Tjoumakaris S, Starke RM, Hasan D, Sidhu N, Singhal S, Hann S, Gonzalez LF, Rosenwasser R, Jabbour P. Endovascular stroke intervention in young patients with large vessel occlusions. Neurosurg Focus. 2014;36:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8192] [Cited by in RCA: 8040] [Article Influence: 268.0] [Reference Citation Analysis (0)] |
| 11. | Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4614] [Cited by in RCA: 4733] [Article Influence: 278.4] [Reference Citation Analysis (0)] |
| 12. | Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, Roine RO, Toni D, Lees KR. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 399] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 13. | de Los Ríos la Rosa F, Khoury J, Kissela BM, Flaherty ML, Alwell K, Moomaw CJ, Khatri P, Adeoye O, Woo D, Ferioli S. Eligibility for Intravenous Recombinant Tissue-Type Plasminogen Activator Within a Population: The Effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke. 2012;43:1591-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Hassan AE, Chaudhry SA, Grigoryan M, Tekle WG, Qureshi AI. National trends in utilization and outcomes of endovascular treatment of acute ischemic stroke patients in the mechanical thrombectomy era. Stroke. 2012;43:3012-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Chalouhi N, Dressler JA, Kunkel ES, Dalyai R, Jabbour P, Gonzalez LF, Starke RM, Dumont AS, Rosenwasser R, Tjoumakaris S. Intravenous tissue plasminogen activator administration in community hospitals facilitated by telestroke service. Neurosurgery. 2013;73:667-671; discussion 671-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 17. | Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, Akhtar N, Orouk FO, Salam A, Shuaib A. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 500] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 18. | Linfante I, Llinas RH, Selim M, Chaves C, Kumar S, Parker RA, Caplan LR, Schlaug G. Clinical and vascular outcome in internal carotid artery versus middle cerebral artery occlusions after intravenous tissue plasminogen activator. Stroke. 2002;33:2066-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Brommer EJ, van Bockel JH. Composition and susceptibility to thrombolysis of human arterial thrombi and the influence of their age. Blood Coagul Fibrinolysis. 1992;3:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Fulgham JR, Ingall TJ, Stead LG, Cloft HJ, Wijdicks EF, Flemming KD. Management of acute ischemic stroke. Mayo Clin Proc. 2004;79:1459-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, Watson T, Goyal M, Demchuk AM. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 543] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 22. | Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. The rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3-13. [PubMed] |
| 23. | del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke. 1998;29:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Ernst R, Pancioli A, Tomsick T, Kissela B, Woo D, Kanter D, Jauch E, Carrozzella J, Spilker J, Broderick J. Combined intravenous and intra-arterial recombinant tissue plasminogen activator in acute ischemic stroke. Stroke. 2000;31:2552-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 1966] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 26. | Ringer AJ, Qureshi AI, Fessler RD, Guterman LR, Hopkins LN. Angioplasty of intracranial occlusion resistant to thrombolysis in acute ischemic stroke. Neurosurgery. 2001;48:1282-1288; discussion 1282-1288. [PubMed] |
| 27. | Hayakawa M, Yamagami H, Sakai N, Matsumaru Y, Yoshimura S, Toyoda K. Endovascular treatment of acute stroke with major vessel occlusion before approval of mechanical thrombectomy devices in Japan: Japanese Registry of Neuroendovascular Therapy (JR-NET) and JR-NET 2. Neurol Med Chir (Tokyo). 2014;54:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Levy EI, Rahman M, Khalessi AA, Beyer PT, Natarajan SK, Hartney ML, Fiorella DJ, Hopkins LN, Siddiqui AH, Mocco J. Midterm clinical and angiographic follow-up for the first Food and Drug Administration-approved prospective, Single-Arm Trial of Primary Stenting for Stroke: SARIS (Stent-Assisted Recanalization for Acute Ischemic Stroke). Neurosurgery. 2011;69:915-920; discussion 920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Roth C, Papanagiotou P, Behnke S, Walter S, Haass A, Becker C, Fassbender K, Politi M, Körner H, Romann MS. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke. 2010;41:2559-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Liu KC, Ding D, Starke RM, Geraghty SR, Jensen ME. Intraprocedural retrieval of migrated coils during endovascular aneurysm treatment with the Trevo Stentriever device. J Clin Neurosci. 2014;21:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36:2311-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Ding D, Liu KC. Management strategies for intraprocedural coil migration during endovascular treatment of intracranial aneurysms. J Neurointerv Surg. 2014;6:428-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Gobin YP, Starkman S, Duckwiler GR, Grobelny T, Kidwell CS, Jahan R, Pile-Spellman J, Segal A, Vinuela F, Saver JL. MERCI 1: a phase 1 study of Mechanical Embolus Removal in Cerebral Ischemia. Stroke. 2004;35:2848-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 293] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 874] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 35. | IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2004;35:904-911. [PubMed] |
| 36. | Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 885] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 37. | Spiotta AM, Chaudry MI, Hui FK, Turner RD, Kellogg RT, Turk AS. Evolution of thrombectomy approaches and devices for acute stroke: a technical review. J Neurointerv Surg. 2014;Jan 2; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 38. | Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 701] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 39. | Simon SD, Grey CP. Hydrodynamic comparison of the Penumbra system and commonly available syringes in forced-suction thrombectomy. J Neurointerv Surg. 2014;6:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Frei D, Gerber J, Turk A, McPherson M, Heck D, Hui F, Joseph G, Jahan R, Miskolczi L, Carpenter J. The SPEED study: initial clinical evaluation of the Penumbra novel 054 Reperfusion Catheter. J Neurointerv Surg. 2013;5 Suppl 1:i74-i76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Castaño C, Dorado L, Guerrero C, Millán M, Gomis M, Perez de la Ossa N, Castellanos M, García MR, Domenech S, Dávalos A. Mechanical thrombectomy with the Solitaire AB device in large artery occlusions of the anterior circulation: a pilot study. Stroke. 2010;41:1836-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Leishangthem L, Satti SR. Vessel perforation during withdrawal of Trevo ProVue stent retriever during mechanical thrombectomy for acute ischemic stroke. J Neurosurg. 2014;121:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Miteff F, Faulder KC, Goh AC, Steinfort BS, Sue C, Harrington TJ. Mechanical thrombectomy with a self-expanding retrievable intracranial stent (Solitaire AB): experience in 26 patients with acute cerebral artery occlusion. AJNR Am J Neuroradiol. 2011;32:1078-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Machi P, Costalat V, Lobotesis K, Maldonado IL, Vendrell JF, Riquelme C, Bonafé A. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg. 2012;4:62-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Hann S, Chalouhi N, Starke R, Gandhe A, Koltz M, Theofanis T, Jabbour P, Gonzalez LF, Rosenwasser R, Tjoumakaris S. Comparison of neurologic and radiographic outcomes with Solitaire versus Merci/Penumbra systems for acute stroke intervention. Biomed Res Int. 2013;2013:715170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1001] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 47. | Mokin M, Dumont TM, Veznedaroglu E, Binning MJ, Liebman KM, Fessler RD, To CY, Turner RD, Turk AS, Chaudry MI. Solitaire Flow Restoration thrombectomy for acute ischemic stroke: retrospective multicenter analysis of early postmarket experience after FDA approval. Neurosurgery. 2013;73:19-25; discussion 25-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, Liebeskind DS, Nogueira RG, Arnold M, Sztajzel R. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke. 2013;44:2802-2807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, Liebeskind DS, Smith WS. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 884] [Cited by in RCA: 868] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 50. | Fargen KM, Meyers PM, Khatri P, Mocco J. Improvements in recanalization with modern stroke therapy: a review of prospective ischemic stroke trials during the last two decades. J Neurointerv Surg. 2013;5:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Mendonça N, Flores A, Pagola J, Rubiera M, Rodríguez-Luna D, De Miquel MA, Cardona P, Quesada H, Mora P, Alvarez-Sabín J. Trevo versus solitaire a head-to-head comparison between two heavy weights of clot retrieval. J Neuroimaging. 2014;24:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Turk AS, Spiotta A, Frei D, Mocco J, Baxter B, Fiorella D, Siddiqui A, Mokin M, Dewan M, Woo H. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg. 2014;6:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 53. | Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, Spiotta A, Mokin M, Dewan M, Quarfordt S. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014;6:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 54. | Mpotsaris A, Bussmeyer M, Weber W. Mechanical Thrombectomy with the Penumbra 3D Separator and Lesional Aspiration: Technical Feasibility and Clinical Outcome. Clin Neuroradiol. 2013;24:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 421] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 56. | Ciccone A, Valvassori L, Ponzio M, Ballabio E, Gasparotti R, Sessa M, Scomazzoni F, Tiraboschi P, Sterzi R. Intra-arterial or intravenous thrombolysis for acute ischemic stroke? The SYNTHESIS pilot trial. J Neurointerv Surg. 2010;2:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 57. | Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1417] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 58. | Starke RM, Komotar RJ, Connolly ES. Endovascular therapy in acute ischemic stroke. Neurosurgery. 2013;72:N20-N23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 397] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 60. | Khalessi AA, Fargen KM, Lavine S, Mocco J. Commentary: Societal statement on recent acute stroke intervention trials: results and implications. Neurosurgery. 2013;73:E375-E379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, Boccardi E. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 62. | Mokin M, Khalessi AA, Mocco J, Lanzino G, Dumont TM, Hanel RA, Lopes DK, Fessler RD, Ringer AJ, Bendok BR. Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg Focus. 2014;36:E5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1017] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 64. | Turk A, Magarik JA, Chaudry I, Turner RD, Nicholas J, Holmstedt CA, Chalela J, Hays A, Lazaridis C, Jauch E. CT perfusion-guided patient selection for endovascular treatment of acute ischemic stroke is safe and effective. J Neurointerv Surg. 2012;4:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Chalouhi N, Ghobrial G, Tjoumakaris S, Dumont AS, Gonzalez LF, Witte S, Davanzo J, Starke RM, Randazzo C, Flanders AE. CT perfusion-guided versus time-guided mechanical recanalization in acute ischemic stroke patients. Clin Neurol Neurosurg. 2013;115:2471-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Kan PT, Snyder KV, Yashar P, Siddiqui AH, Hopkins LN, Levy EI. Utility of CT perfusion scanning in patient selection for acute stroke intervention: experience at University at Buffalo Neurosurgery-Millard Fillmore Gates Circle Hospital. Neurosurg Focus. 2011;30:E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Turk AS, Nyberg EM, Chaudry MI, Turner RD, Magarik JA, Nicholas JS, Holmstedt CA, Chalela JA, Hays A, Lazaridis C. Utilization of CT perfusion patient selection for mechanical thrombectomy irrespective of time: a comparison of functional outcomes and complications. J Neurointerv Surg. 2013;5:518-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Spiotta AM, Vargas J, Hawk H, Turner R, Chaudry MI, Battenhouse H, Turk AS. Impact of the ASPECT scores and distribution on outcome among patients undergoing thrombectomy for acute ischemic stroke. J Neurointerv Surg. 2014;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Starke RM, Komotar RJ, Connolly ES. Mechanical clot retrieval in the treatment of acute ischemic stroke. Neurosurgery. 2013;72:N19-N21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |