Published online Oct 16, 2014. doi: 10.12998/wjcc.v2.i10.596
Revised: June 26, 2014
Accepted: July 25, 2014
Published online: October 16, 2014
Processing time: 145 Days and 2.9 Hours
Redo-sternotomy and aortic valve replacement in patients with advanced liver disease is rare and associated with a prohibitive morbidity and mortality. Refractory coagulopathy is common and a consequence of intense activation of the coagulation system that can be triggered by contact of blood with the cardiopulmonary bypass circuitry, bypass-induced fibrinolysis, platelet activation and dysfunction, haemodilution, surgical trauma, hepatic decompensation and hypothermia. Management can be further complicated by right heart dysfunction, porto-pulmonary hypertension, poor myocardial protection, and hepato-renal syndrome. Complex interactions between coagulation/fibrinolysis and systemic inflammatory response syndrome reactions like “post-perfusion-syndrome” also compound haemostatic failure. Given the limited information available for the specific management and prevention of cardiopulmonary bypass-induced haemostatic failure, this report serves to guide the anaesthesia and medical management of future cases of a similar kind. We discuss our multimodal management of haemostatic failure using pharmacological strategies, thromboelastography, continuous cerebral and liver oximetry, and continuous cardiac output monitoring.
Core tip: Cardiac surgery in patients with advanced liver disease is associated with significant morbidity and mortality. Refractory coagulopathy is common and requires a proactive multidisciplinary haemostatic management strategy. Given the limited information available for the specific management and prevention of cardiopulmonary bypass induced haemostatic failure, this report serves to guide the anaesthesia and medical management of future cases of a similar kind. We discuss our multimodal management of haemostatic failure using pharmacological strategies, thromboelastography, continuous cerebral and liver oximetry, and continuous cardiac output monitoring.
- Citation: Weinberg L, Kearsey I, Tjoakarfa C, Matalanis G, Galvin S, Carson S, Bellomo R, McNicol L, McCall P. Haemostatic management for aortic valve replacement in a patient with advanced liver disease. World J Clin Cases 2014; 2(10): 596-603
- URL: https://www.wjgnet.com/2307-8960/full/v2/i10/596.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i10.596
Redo-sternotomy and aortic valve replacement (AVR) in patients with advanced liver disease is rare and associated with a prohibitive morbidity and mortality. Refractory coagulopathy is common and a consequence of intense activation of the coagulation system that can be triggered by contact of blood with the cardiopulmonary bypass (CPB) circuitry, CPB-induced fibrinolysis, platelet activation and dysfunction, haemodilution, surgical trauma, hepatic decompensation and hypothermia. Management can be further complicated by right heart dysfunction, porto-pulmonary hypertension, poor myocardial protection, and hepato-renal syndrome. Complex interactions between coagulation/fibrinolysis and systemic inflammatory response syndrome (SIRS) reactions like “post-perfusion-syndrome” also compound haemostatic failure.
We present a patient with critical aortic stenosis who underwent redo-sternotomy and AVR prior to being listed for orthotopic liver transplantation. In this context, there is little information on the specific management of CPB-induced haemostatic failure. Therefore, we discuss our multimodal management of haemostatic failure using pharmacological strategies, thromboelastography (TEG), continuous cerebral and liver oximetry, and continuous cardiac output monitoring.
A 46-year-old male (weight 68 kg, height 183 cm) presented to our institution with acute pulmonary oedema secondary to severe aortic stenosis. The patient consented for a redo-sternotomy and AVR, with an estimated perioperative mortality of 50%. Previous cardiac history included open valvotomy via median sternotomy for a congenital calcified bicuspid aortic valve at age 6. The patient had a 10-year history of chronic liver disease secondary to alcohol abuse, with a Child Pugh Score of 8 (Child Class B), and a Model for End-Stage Liver Disease (MELD) Score of 12. The liver disease was further complicated by severe portal hypertension with ascites, thrombocytopaenia, oesophageal varices and portal hypertensive gastropathy. Two years prior, he underwent an emergency laparotomy for bleeding umbilical varices, which required intensive care unit (ICU) admission and an 8-unit red blood cell transfusion for hemorrhagic shock.
On this admission a transthoracic echocardiogram revealed preserved systolic left and right ventricular function, a severely calcified bicuspid valve (aortic valve area: 0.7 cm2; mean aortic valve pressure gradient of 60 mmHg), moderate aortic regurgitation, mild mitral regurgitation, moderate pulmonary hypertension and a dilated ascending aorta (5.4 cm). Other cardiovascular risk factors included IgA nephropathy (creatinine 110 μmol/L, estimated glomerular filtration rate 73 mL/min per 1.73 m2). There was no history of smoking or diabetes. The pulmonary oedema settled with conservative medical therapy. A coronary angiogram and right heart catheter study revealed no occlusive coronary artery disease, with a cardiac index of 3.4 L/min per square meter and a pulmonary artery pressure of 71/30 mmHg (mean 33 mmHg). Preoperative investigations including TEG are summarised in Tables 1, 2 and 3. A detailed perioperative haemostatic coagulation strategy was formulated by a team composed of anaesthetist, haematologist, cardiac surgeon and intensivist.
| Ref. ranges | Pre-operative | Pre-CPB | Rewarming Haemostatic intervention: FFP 15 mg/kg | Immediately post separation from CPB Haemostatic intervention: Protamine 500 mg; DDAVP 0.3 mg/kg; Concentrated fibrinogen 4 g;Platelets 2 pooled doses; 2 units packed RBC | 15 min post separation from CPB Haemostatic intervention: Prothrombine × 1000 IU; then continuous infusion at 100 IU /h | 30-min post separation from CPB Haemostatic intervention: Nil | Arrival intensive care unit | |
| R (min) | 4-8 | 6.4 | 5.0 | 6.7 | 6.9 | 28.9 | 7.8 | 6.7 |
| K (min) | 0-4 | 2.0 | 1.8 | 1.8 | 1.8 | 4.9 | 1.5 | 1.6 |
| Angle (deg) | 47-74 | 62.7 | 63.5 | 65.6 | 65.2 | 49.6 | 68.5 | 65.5 |
| MA (mm) | 54-72 | 53.7 | 61.9 | 61.3 | 57.6 | 71.8 | 69.0 | 70.1 |
| LY30 (%) | 0-8 | 0.7 | 0.3 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 |
| INR | - | 1.3 | 1.2 | 1.4 | 1.7 | 1.5 | 1.4 | 1.4 |
| PT | 11-15 s | 13 s | 14 s | 15 s | 19 h | 17 h | 16 h | 16 h |
| APPT | 22-38 s | 36 s | 39 h | > 200 h | 49 h | 50 h | 45 h | 38 s |
| Fib clauss | 2.0-4.0 g/L | 2.3 g/L | 1.3 L | 2.6 g/L | 2.0 g/L | 1.6 L | 1.8 g/L | |
| D-dimer | < 0.23 mg/L | 1.05 h | Not measured | 0.94 h | 1.15 mg/L | Not measured | Not measured | |
| Hb | 130-180/L | 90 L | 83 L | 73 L | 55 L | 85 L | Not measured | 71 L |
| WBC | 4.0-11.0 × 109 | 3.9 L | 4.2 × 109 | 10.8 × 109 | 8.2 × 109 | 11.7 h | Not measured | 9.4 × 109 |
| Platelets | 150-400 × 109 | 76 L | 64 L | 57 L | 140 L | 120 L | Not measured | 73 L |
| Ref. ranges | Pre-operation | Pre-cardiopulmonary bypass | Rewarming | Post separation | Closure | Post-op day 1 | Post-op day 2 (venous) | |
| pH | 7.35-7.45 | 7.341 | 7.321 | 7.371 | 7.241 | 7.341 | 7.341 | 7.331 |
| pCO2 (mmHg) | 35-45 | 37 | 38 | 35 | 502 | 42 | 39 | 45 |
| pO2 (mmHg) | 80-110 | 105 | 2302 | 3882 | 3852 | 4042 | 1112 | 331 |
| HCO3- (mmol/L) | - | 19 | 19 | 20 | 20 | 22 | 20 | 23 |
| Base excess (mmol/L) | -3/+3 | -61 | -61 | -41 | -61 | -3 | -51 | -2 |
| O2 sat (%) | > 94 | 98 | 100 | 100 | 100 | 100 | 100 | 561 |
| Na+ (mmol/L) | 135-148 | 1321 | 1322 | 136 | 138 | 138 | 138 | 1301 |
| K+ (mmol/L) | 3.5-5.3 | 4.0 | 4.0 | 4.9 | 3.9 | 3.9 | 4.6 | 4.5 |
| Cl- (mmol/L) | 95-106 | 1071 | 1072 | 106 | 1082 | 1082 | 106 | 99 |
| Ionised Ca2+ (mmol/L) | 1.13-1.32 | 1.16 | 1.071 | 0.921 | 0.831 | 1.061 | 1.101 | 1.101 |
| Haemoglobin (g/L) | 120-180 | 801 | 781 | 711 | 771 | 861 | 711 | 631 |
| Glucose (mmol/L) | 0.0-5.0 | 5.92 | 6.32 | 8.32 | 6.22 | 3.81 | 8.02 | 8.92 |
| Lactate (mmol/L) | 3.9-5.8 | 0.9 | 0.6 | 4.02 | 2.92 | 2.02 | 1.5 | 1.3 |
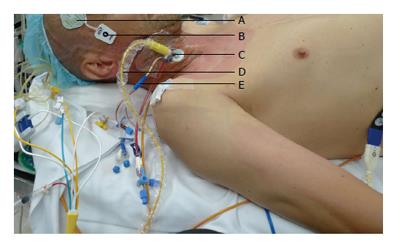
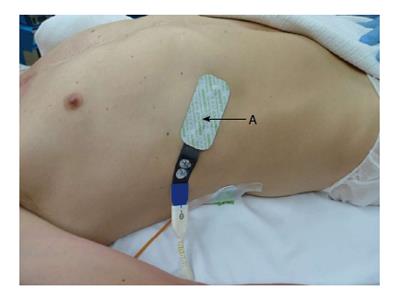
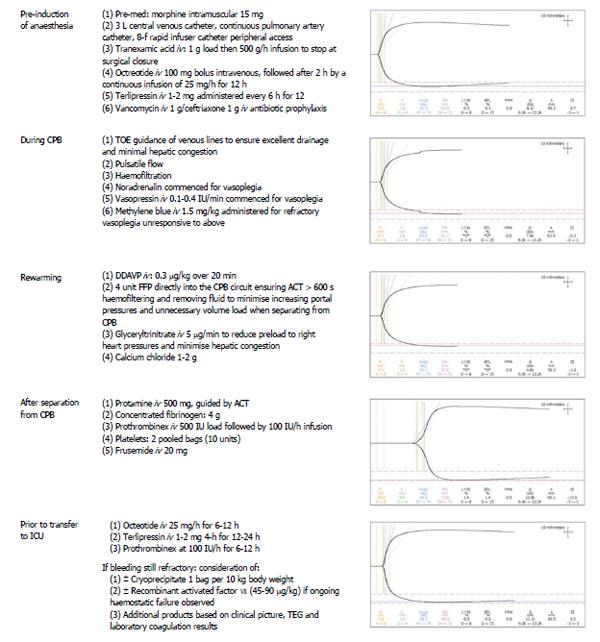
The day before surgery, terlipressin [1 mg intravenous (iv) every 6 h] was commenced. Prior to induction of anaesthesia, an 8-French Rapid Infuser Catheter (Arrow) was inserted into each arm. Invasive monitoring included a 20 Gauge arterial line, 4-lumen central venous catheter, continuous cardiac output and continuous mixed venous oximetry measured with a fiberoptic pulmonary artery catheter (Edwards Lifesciences, Irvine CA) (Figure 1). External defibrillator pads were applied as a safety precaution. Bispectral index monitoring and cerebral and hepatic tissue oxygenation (Invos, Somanetics®) were measured with a cerebral/somatic oximeter, by placing disposable transducers over the right and left forehead (Figure 1), and on the skin overlying the lower right costal margin (Figure 2). The oximeters provided real-time monitoring of brain and liver oxygen saturations, measuring oxygen consumption and delivery. This allowed for detection and correction of cerebral and hepatic oxygen desaturation to optimise haemodynamic intervention. Tranexamic acid (1 g iv load then 500 g/h infusion) was commenced to minimize fibrinolysis during and after CPB. Octreotide (100 mcg bolus, then 25 mcg/h) was commenced to control portal hypertension and minimise hepatic ischaemia reperfusion injury from CPB. Vancomycin (1 g iv) and ceftriaxone (1 g iv) were administered for antimicrobial prophylaxis.
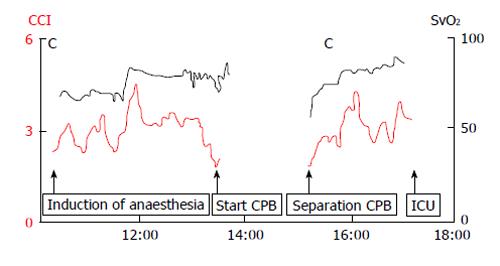
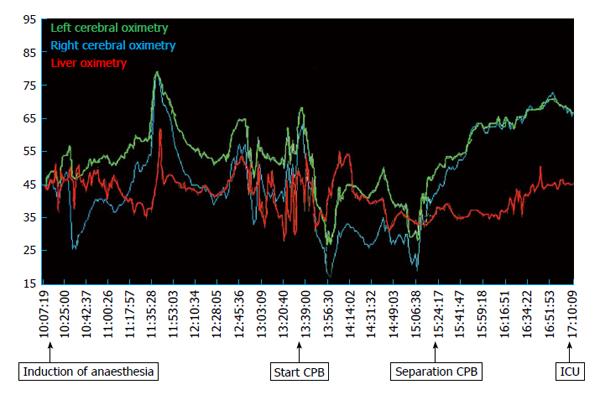
Redo-sternotomy was performed using an oscillating saw while lifting up the sternal wires. Dense adhesions of the right ventricle and posterior table of the sternum precluded access to the heart for central venous cannulation. Consequently, the femoral artery and vein were cannulated and the venous cannula carefully positioned using transoesophageal echocardiography guidance in the right atrium. After careful dissection around the heart and full heparinisation, the standard on-pump AVR technique was applied. A second venous cannula was added via the superior vena cava to the venous circuit to allow venous drainage, further minimizing hepatic congestion. After aortic cross clamp, pulsatile CPB was established, complete with haemofiltration to prevent fluid overload and maintain electrolyte neutrality. During CPB the patient was severely vasoplegic requiring escalating doses of noradrenaline (20 μg/min iv) and vasopressin (0.4 IU/min iv) to maintain a mean arterial pressure of 50 mmHg. Optimal pump flow rates and vasopressor use were guided by cerebral and liver oximetry. There was excellent correlation between cardiac output, mixed venous saturations and cerebral and liver oximetry throughout the case (Figures 3 and 4). In response to progressive refractory vasoplegia, methylene blue (1 mg/kg iv) was administered, which rapidly re-established an acceptable mean arterial pressure. The noradrenaline and vasopressin requirements were weaned to 3 μg/min and 0.05 IU/min respectively. A 25 mm Mitroflow® aortic pericardial valve (Sorin, Milan, Italy) was inserted without complication.
Prior to separation from CPB, a rewarming heparinase TEG was performed (Table 1, Figure 5). Desmopressin acetate (0.3 mcg/kg iv over 20 min) was administered to increase the plasma levels of factor VIII and von Willebrand factor to minimize post-operative blood loss. Based on the rewarming TEG (Table 2 and Figure 5), fresh frozen plasma (15 mg/kg) was added to the CPB circuit to avoid volume overload and right ventricular distension during bypass separation. Glyceryl trinitrate (5 μg/min iv), and frusemide (20 mg iv) were administered to further reduce right ventricular preload and hepatic congestion.
After successful separation from CPB, haemostatic management focused on minimizing intraoperative bleeding and maintaining normothermia. Protamine (500 mg iv) was given to reverse the effects of heparin and correct activated clotting time to baseline values, followed by two bags of pooled platelets and iv administration of concentrated fibrinogen (4 g iv) (Riastap®, iv Behring, Australia). The dose of fibrinogen was calculated according to the patient’s body weight (68 kg) and his estimated blood volume (4.8 L). Based on a preoperative haemoglobin of 9.8 g/L, a haematocrit of 30% of plasma volume (3.4 L), and a preoperative fibrinogen level of 2.5 g/L, we calculated that a dose of 4 g of fibrinogen would be needed to increase plasma fibrinogen levels by an estimated 1.2 g/L. With haemodilution on bypass, we expected the fibrinogen to fall by approximately 1-1.5 g/L. Following administration of concentrated fibrinogen, a heparinase TEG revealed significant prolongation of the R-time, confirming an underlying coagulopathy (Figure 5). Human prothrombin complex® (500 IU iv bolus, then 100 IU/h iv infusion) (CSL Behring, Australia) was administered, which corrected the R-time and improved haemostasis (Figure 5). Calcium chloride (1-2 g iv) was also administered. The total CPB time was 141 min and aortic cross-clamp time 61 min. Temporary epicardial pacing wires were not used to avoid the small risk of cardiac bleeding on wire removal additional. A topical haemostatic matrix (FLOSEAL™, Baxter, Pty) was used to control bleeding from the suture lines, and thrombin dried powder (GELFOAM®, Baxter, Pty) was applied to the bone marrow of the sternum, which allowed sternal closure with minimal bleeding. Mediastinal and bilateral pleural drains were placed so that volume losses could be measured in ICU, and collection of blood around the heart avoided during the postoperative period.
In ICU, the octreotide (25 mcg/h iv) and prothrombinex (100 IU/h iv) infusions were continued for 8 h. Haemodynamic stability was maintained and the noradrenaline and vasopressin infusions weaned after 6 h, and the patient was extubated. Terlipressin (1 mg iv) was continued every 6 h for a further 24 h. The patient was transferred to the ward the following day, and discharged home ten days later without complications. There were no further requirements for coagulation or blood product intervention. Postoperatively, renal, haematological and liver function tests remained stable, and are summarised in Tables 1-3.
Three months post discharge, and at the time of writing, the patient continues to make satisfactory cardiac progress and is currently awaiting liver transplantation.
This case illustrates that redo-sternotomy and aortic valve AVR in the setting of advanced liver disease is feasible but requires careful planning. The central management decisions for such cases include to either (1) replace the valve first and then proceed with liver transplantation at a later date; (2) offer a combined procedure, i.e., AVR and liver transplantation simultaneously; and (3) proceed with liver transplantation first, and then replace the valve at a later stage. In the case described here, after extensive multidisciplinary discussion a consensus was reached that the risks of liver transplantation in the setting of uncorrected symptomatic severe aortic stenosis were prohibitive. In view of the bicuspid valve and dilated ascending aorta, a transcatheter AVR was not a consideration. A combined AVR and liver transplant was considered but there were concerns that there may be further cardiac decompensation during the waiting period. Given that the patient was progressively symptomatic, a redo-sternotomy and AVR was considered to afford the best chance of survival, with activation for liver transplantation initiated at a later stage if the outcome was successful.
As shown in this case, redo cardiac surgery provides several technical challenges that distinguish it from primary cardiac surgery. These obstacles include repeat sternotomy, injury to the heart during dissection, quality and availability of conduits if required, a calcified ascending aorta, and more-advanced coronary disease involving the native vessels. As a result, operative mortality in most reoperations is 3 to 5 times that for a primary AVR. Adding in the ensuing complications of advanced liver disease, perioperative mortality increases with an estimated perioperative risk of mortality of 50%[1]. Each patient’s condition and presentation is unique, and thus requires individualized management delivered by a multidisciplinary team. Consideration must be given to the sequence of procedures, cardiac surgical technique, and management of anticoagulation.
Combined cardiac and liver transplantation was first reported by Starzl et al[2] in 1984, but has remained uncommon because of the unique medical and surgical challenges it poses . In two descriptive reports of outcomes in patients with advanced liver cirrhosis undergoing cardiac surgery[3,4], hepatic decompensation, respiratory and renal failure, gastrointestinal haemorrhagic events, sepsis and mediastinitis were among the most common postoperative complications. The association of MELD scores and Child-Turcotte-Pugh classification, and adverse outcomes is less clear. In the study by Filsoufi et al[4], mortality rate increased significantly according to the Child-Turcotte-Pugh classification (class A, 10%; B, 18%; and C, 67%). The reported mortality of redo cardiac surgery was approximately 50%[3]. Similarly, the rate of complications was higher in class B (50%) and C (100%) compared to class A (20%). Suman et al[5] reported that a cutoff Child-Pugh score > 7 had a sensitivity and specificity of 86% and 92% for mortality, although there was no association between mortality and MELD scores. In contrast, Morimoto et al[3] reported that Child-Pugh class score did not correlate with hospital mortality, although MELD score was significantly higher in patients who died immediately post cardiac surgery. To date, there have been several reports of combined AVR and liver transplantation[6]. Postoperative outcomes are variable; the majority of cases have been successful however, mortality due to clotting disturbances has also been reported. As a result, careful preoperative preparation must be conducted in such highly complex cases to prevent catastrophic outcomes.
As seen in this case, a common yet serious complication of CPB is vasoplegic syndrome, a post-perfusion syndrome characterised by low systemic vascular resistance, significant hypotension, and a high cardiac output. It has an incidence of 5%-25% and a mortality rate as high as 25%[7]. In this case, we used the standard first line vasoactive treatment (noradrenaline and vasopressin) to maintain a mean arterial pressure of 50 mmHg[8]. However methylene blue was required during CPB to reduce the severity of vasoplegia. Use of methylene blue had been used effectively for the treatment of refractory vasoplegia in two randomised control trials[9,10], acting through its inhibitory effect on cyclic guanosine 3’,5’-monophosphate-mediated vasodilatation. Despite restoring vascular tone intraoperatively, discontinuation of vasopressin has been associated with postoperative refractory vasoplegia, therefore in the case described here, vasopressin was for continued for 6 h postoperatively.
AVR performed in the context of advanced liver disease added an additional layer of complexity in preventing further decline of hepatic and renal function. The patient’s history of significant portal hypertension justified the use of both terlipressin and octreotide to prevent variceal beeding[11-13]. Terlipressin has also been shown to improve hepatorenal syndrome, thought to be due to arteriolar vasoconstriction in the splanchnic area, which was an important consideration for the patient’s underlying IgA nephropathy. Its effects are mediated via V1 receptors on vascular smooth muscle[13]. In animal models, octreotide has been shown to improve hepatic ischaemia-reperfusion injury by down-regulating inflammatory cytokines (tumor necrosis factor alpha and interleukin-1 beta) and inhibition of hepatocellular apoptosis[14], and in this case, served an added benefit when separating from bypass.
Coagulopathy is a frequent occurrence during CPB and is due to a number of factors including excessive fibrinolysis, platelet dysfunction, coagulation factor consumption, and coagulation factor dilution from intravascular volume replacement. We used a variety of multimodal pharmacological agents to prevent intraoperative and postoperative bleeding. Hypofibrinogenemia is common in cardiac surgery, which was minimized preoperatively with tranexamic acid, and intraoperatively with concentrated fibrinogen[15,16]. Desmopressin acetate[17,18], Human Prothrombin-X complex[19] and protamine were also implemented as described previously.
In this case, we employed several haemostatic and haemodynamic monitoring methods to guide our management. TEG, commonly used in cardiac surgery, is a useful tool in denoting a patient’s clotting profile at landmark time points to influence specific pharmacologic decisions[20,21]. Figure 5 summarizes consecutive TEG readings and the subsequent interventions undertaken. It should also be noted that TEG requires trained personnel to operate and therefore poses as a limiting factor for its use[20]. Additional haemodynamic monitoring included pulmonary artery catheter sampling of mixed venous blood (SvO2) and tissue oximetry. Intraoperatively, we were primarily concerned about the key factors that influence oxygen delivery, namely haemoglobin, oxygenation, and cardiac output. Continuous liver, cerebral and mixed venous oximetry enabled immediate detection of adverse changes, prompting correction and subsequent visualization of improvements of haemodynamic trends. Continuous recordings of cardiac output and global oxygenation status are presented in Figure 3. The brain and liver tissue oxygenation tracings are shown in Figure 4. In the context of low liver oximetry, in addition to the aforementioned factors that influence tissue oxygenation, hepatic congestion secondary to the outflow obstruction was also carefully monitored. Then, depending on the determined underlying cause, suitable corrections were made in the form of red cell transfusion, adjustment pump flow rates, and ensuring adequate venous drainage at all times. Although hepatic oximetry is predominantly used in the paediatric setting[22,23], we justified its use to intensively monitor the already compromised liver, and guide therapy as above. Interestingly, the liver oximeter tracing tracked the cerebral oximeter tracing very accurately (Figure 4), providing reassurance of continual intact hepatic perfusion.
In conclusion, we report a case of AVR in a patient with advanced liver disease. Given the limited information available for specific management and prevention of haemostatic failure, this report serves to guide future cases of a similar kind.
A 46-year-old male with a history of chronic liver disease secondary to alcohol abuse, presents with acute pulmonary oedema secondary to left ventricular failure.
Severe aortic stenosis.
Non cardiogenic causes of pulmonary oedema include pulmonary contusion, acute respiratory distress syndrome, transfusion-related acute lung injury, aspiration, hypertensive crisis, upper airway obstruction, and neurogenic causes (seizures, intracranial haemorrhage).
Plasma creatinine 110 μmol/L; albumin 31 g/L; bilirubin 40 μmol/L; haemoglobin 90 g/L; prothrombin time 1.3 s; platelets 76 (× 109).
Transthoracic echocardiogram a severely calcified bicuspid valve, with an aortic valve area of 0.7 cm2; mean aortic valve pressure gradient of 60 mmHg with moderate pulmonary hypertension.
The patients underwent redo-aortic valve replacement requiring aggressive haemostatic therapy for coagulopathy and refractory vasoplegia.
Combined cardiac surgery in patients with advanced liver disease.
Cardiac surgery in patients with advanced liver disease is associated with significant morbidity and mortality. Refractory coagulopathy is common and requires a proactive multidisciplinary haemostatic management strategy.
Weinberg et al present an interesting and complex case report of a patient with critical aortic stenosis and advanced liver disease who underwent redo-sternotomy and aortic valve replacement prior to being listed for orthotopic liver transplantation.
P- Reviewer: Can M, Petix NR S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Nemati MH, Astaneh B, Zamirian M. Aortic valve replacement in a patient with liver cirrhosis and coagulopathy. Gen Thorac Cardiovasc Surg. 2008;56:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Starzl TE, Iwatsuki S, Shaw BW, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR. Analysis of liver transplantation. Hepatology. 1984;4:47S-49S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Morimoto N, Okada K, Okita Y. Results of cardiac surgery in advanced liver cirrhosis. Gen Thorac Cardiovasc Surg. 2013;61:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Filsoufi F, Salzberg SP, Rahmanian PB, Schiano TD, Elsiesy H, Squire A, Adams DH. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl. 2007;13:990-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Suman A, Barnes DS, Zein NN, Levinthal GN, Connor JT, Carey WD. Predicting outcome after cardiac surgery in patients with cirrhosis: a comparison of Child-Pugh and MELD scores. Clin Gastroenterol Hepatol. 2004;2:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Diaz GC, Renz JF, Nishanian E, Kinkhabwala M, Emond JC, Wagener G. Anesthetic management of combined heart-liver transplantation. J Cardiothorac Vasc Anesth. 2007;21:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Gomes WJ, Carvalho AC, Palma JH, Teles CA, Branco JN, Silas MG, Buffolo E. Vasoplegic syndrome after open heart surgery. J Cardiovasc Surg (Torino). 1998;39:619-623. [PubMed] |
| 8. | Fischer GW, Levin MA. Vasoplegia during cardiac surgery: current concepts and management. Semin Thorac Cardiovasc Surg. 2010;22:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Maslow AD, Stearns G, Butala P, Schwartz CS, Gough J, Singh AK. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg. 2006;103:2-8, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kücükarslan N, Günay C, Demirkilic U, Tatar H. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005;79:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Fayed N, Refaat EK, Yassein TE, Alwaraqy M. Effect of perioperative terlipressin infusion on systemic, hepatic, and renal hemodynamics during living donor liver transplantation. J Crit Care. 2013;28:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Ludwig D, Schädel S, Brüning A, Schiefer B, Stange EF. 48-hour hemodynamic effects of octreotide on postprandial splanchnic hyperemia in patients with liver cirrhosis and portal hypertension: double-blind, placebo-controlled study. Dig Dis Sci. 2000;45:1019-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Mukhtar A, Salah M, Aboulfetouh F, Obayah G, Samy M, Hassanien A, Bahaa M, Abdelaal A, Fathy M, Saeed H. The use of terlipressin during living donor liver transplantation: Effects on systemic and splanchnic hemodynamics and renal function. Crit Care Med. 2011;39:1329-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Yang J, Sun H, Takacs P, Zhang Y, Liu J, Chang Y, Candiotti KA. The effect of octreotide on hepatic ischemia-reperfusion injury in a rabbit model. Transplant Proc. 2013;45:2433-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Rahe-Meyer N, Hanke A, Schmidt DS, Hagl C, Pichlmaier M. Fibrinogen concentrate reduces intraoperative bleeding when used as first-line hemostatic therapy during major aortic replacement surgery: results from a randomized, placebo-controlled trial. J Thorac Cardiovasc Surg. 2013;145:S178-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Rahe-Meyer N, Solomon C, Hanke A, Schmidt DS, Knoerzer D, Hochleitner G, Sørensen B, Hagl C, Pichlmaier M. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 200] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Steinlechner B, Zeidler P, Base E, Birkenberg B, Ankersmit HJ, Spannagl M, Quehenberger P, Hiesmayr M, Jilma B. Patients with severe aortic valve stenosis and impaired platelet function benefit from preoperative desmopressin infusion. Ann Thorac Surg. 2011;91:1420-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Wademan BH, Galvin SD. Desmopressin for reducing postoperative blood loss and transfusion requirements following cardiac surgery in adults. Interact Cardiovasc Thorac Surg. 2014;18:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Song HK, Tibayan FA, Kahl EA, Sera VA, Slater MS, Deloughery TG, Scanlan MM. Safety and efficacy of prothrombin complex concentrates for the treatment of coagulopathy after cardiac surgery. J Thorac Cardiovasc Surg. 2014;147:1036-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Gorton H, Lyons G. Is it time to invest in a thromboelastograph? Int J Obstet Anesth. 1999;8:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Salooja N, Perry DJ. Thrombelastography. Blood Coagul Fibrinolysis. 2001;12:327-337. [PubMed] |
| 22. | Hampton DA, Schreiber MA. Near infrared spectroscopy: clinical and research uses. Transfusion. 2013;53 Suppl 1:52S-58S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Schulz G, Weiss M, Bauersfeld U, Teller J, Haensse D, Bucher HU, Baenziger O. Liver tissue oxygenation as measured by near-infrared spectroscopy in the critically ill child in correlation with central venous oxygen saturation. Intensive Care Med. 2002;28:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |