Published online Oct 16, 2014. doi: 10.12998/wjcc.v2.i10.591
Revised: July 2, 2014
Accepted: July 25, 2014
Published online: October 16, 2014
Processing time: 163 Days and 0.4 Hours
Extra-adrenal chromaffin cell-related tumours or paragangliomas are rare, especially in the bladder. In this article, we reported three different clinical cases of bladder paraganglioma, followed by a review of current literature on the pathophysiology and management of bladder paraganglioma. Case 1 involved a 23 years old female patient who complained of a 10-year history of micturition-related headaches, palpitations and diaphoresis; while in case 2, a 58 years old female patient presented with history of painless haematuria and an incidentally diagnosis of a functioning paraganglioma during endoscopic transurethral resection of bladder tumour; and lastly in case 3, a 54 years old male renal transplant recipient was referred to the urology outpatient with a suspicious bladder mass found incidentally on routine transplant workshop.
Core tip: Bladder paraganglioma is a rare condition and patients can present with various clinical presentations. Biochemical profiling and nuclear imaging study can assist in the identification of this lesion. Preoperative care with volume hydration and adrenergic blockade are often necessary and surgery remains the only cure for these patients.
- Citation: Ranaweera M, Chung E. Bladder paraganglioma: A report of case series and critical review of current literature. World J Clin Cases 2014; 2(10): 591-595
- URL: https://www.wjgnet.com/2307-8960/full/v2/i10/591.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i10.591
Paragangliomas are rare tumours that arises from extra-adrenal paraganglia and consists of specialized neural crest-derived cells (catecholamine-secreting chromaffin cells)[1]. Of all chromaffin cell-related tumours, paraganglioma accounts for less than a quarter of cases[1,2]. The sympathetic paraganglia are symmetrically distributed along the paravertebral axis and small sympathetic paraganglia can also be found in other organs such as the bladder. Primary paraganglioma of the urinary bladder is very rare making up less than 0.05% of all bladder malignancy. Paragangliomas can present with clinical symptoms secondary to catecholamine hypersecretion or mass effect, incidental finding on radiographic imaging, and/or on routine family screening for hereditary paraganglioma. We explore three different clinical cases of bladder paraganglioma that were treated at our institution.
A 23 years old female was referred by her general practitioner with history of urinary urgency and an incidental finding of bladder mass on urinary tract ultrasound. When enquired further, she described a long-standing history of episodic severe throbbing headaches lasting few minutes, dating back to as early as the age of 12 years. These paroxysmal attacks of headache coincided with her bladder emptying and over the last few years, she also experienced palpitations, nausea, sweat and facial pallor post-micturition. In addition, she recalled the presence of microscopic hematuria in her urine dipstick since the age of 12 years. She has been diagnosed with borderline hypertension in her late teens but is not on anti-hypertensive medication. There was no family history to suggest a hereditary endocrine disorder.
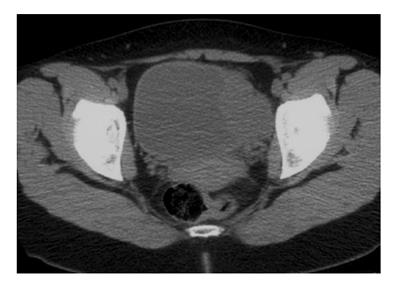
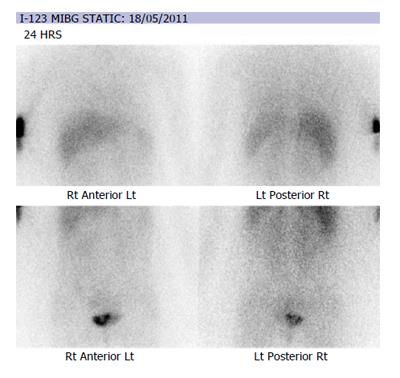
Urinary tract ultrasonography revealed a large vascular soft tissue mass on the left bladder wall and her urine cytology revealed mildly atypical urothelial cells in two out of three samples. There was a marked elevation of urinary noradrenaline (4748 nmol/dL, Reference: [50-600]) and its metabolites on 24 h urine collection. Plasma metanephrine was also significantly elevated (8500 pmol/L, reference < 900 pmol/L). Staging computed topography of the abdomen and pelvis showed a 6.2 cm × 4 cm × 4.9 cm solid and enhancing, loculated mass near to the left vesicoureteric junction (Figure 1). Following consultation with the endocrinologist, alpha (α)-adrenergic and beta (β)-adrenergic blockade was achieved with a combination of phenoxybenzamine and metoprolol for a minimum of 2 wk prior to surgery. During this time, further imaging with Iodine-123-meta-iodobenzylguanidine (I-123 MIBG) scintiscan confirmed the presence of bladder phaeochromocytoma without metastatic disease (Figure 2).
A formal rigid cystoscopy, left retrograde pyelogram and examination under anesthesia were performed and showed a firm, irregular, well circumscribed lesion in the left bladder that was not fixed to the pelvis and a normal distal left ureter configuration. She was hypertensive intraoperative and required α-adrenergic blockade with phentolamine. Discussion ensued about her condition and she underwent partial cystectomy 2 wk later with volume hydration and antihypertensive medication. A combination of adrenergic blockade with phentolamine, esmolol and metaraminol were utilised intraoperatively during her partial cystectomy.
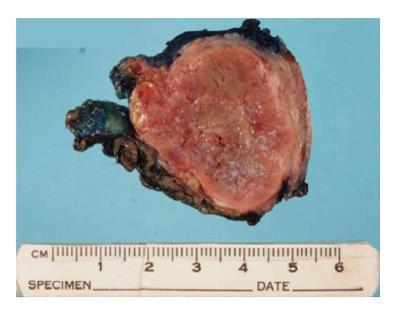
The histopathology of the bladder wall specimen sections showed a 35 mm × 32 mm well-circumscribed, lobulated, red-brown tumour (Figure 3). Microscopically, the tumour was composed of nests of cells with eosinophilic cytoplasm and round nuclei with vesicular chromatin. It was encapsulated with no evidence of extracapsular invasion and surgical margins were clear (Figure 4). The tumour cells stained positive for chromogranin, patchy for S-100 and negative for cytokeratin consistent with urinary bladder paraganglioma.
Further immunohistochemistry on the tumour specimen revealed the tumour was negative for succinate dehydrogenase subunit B (SDHB). She was subsequently discharged and remained asymptomatic at 36 mo of follow up.
A 58 years old female underwent a routine uneventful resection of bladder mass found during investigation for painless macroscopic haematuria. The histopathology of this mass showed solid nests of plump epithelial cells with granular to foamy cytoplasm and enlarged, moderate pleomorphic nuclei. Immunohistochemical staining was strongly positive for chromogranin, neuron-specific enolase (NSE), synaptophysin and protein gene product 9.5, consistent with a neuroendocrine tumour. On retrospect history, she had been taking atenolol and oxazepam for her moderate hypertension and anxiety disorder. Given that the patient’s younger sister had a history of poorly controlled hypertension, both sisters underwent genetic testing and von Hippel Lindau (VHL), SDHB and succinate dehydrogenase screening, but were negative.
Further investigations showed an elevated creatinine ratio of urinary normetanephrine (0.40 mmol/mmol creatinine) and metanephrine (0.13 mmol/mmol creatinine), and an increased 24-h excretion of normetanephrine (3.2 μmol/24 h). Nuclear imaging study with I-123 MIBG scan showed a discrete round focus on the left side of the bladder with no further evidence of metastases.
The patient was commenced on phenoxybenzamine and propranolol preoperatively for 2 wk and underwent open partial cystectomy and re-implantation of her left ureter. Intra-operatively the patient remained haemodynamically stable. Histopathology confirmed bladder paraganglioma with multiple nodules found predominately within the muscularis propria but not extending into the peri-vesicle soft tissue and a clear of the surgical margins. The tumour showed focal moderate nuclear pleomorphism with no lymphovascular invasion. She remained asymptomatic with normal imaging test at 60 m of follow up.
A 54 years old male renal transplant recipient for end-stage renal failure (secondary to Alport’s syndrome) was referred for investigation of an asymptomatic bladder mass. After having undergone a left sided radical nephrectomy for papillary renal cell carcinoma 12 mo earlier, this bladder mass was discovered on surveillance urinary tract ultrasonography (Figure 5). He underwent routine cystoscopy which revealed a submucosal polyp at the anterior bladder suspicious for urothelial tumour and this bladder “tumour” was completely resected.
Histological examination revealed tumour cells arranged in nested architecture with circumscribed margin and focal areas of smooth muscle invasion. Interestingly the immunohistochemistry staining of the tumour cells were positive for synaptophysin and chromogranin but negative for cytokeratin.
The patient subsequently underwent an I-123 MIBG scan and metastatic disease was excluded. Serum and urinary catecholamines were negative too. Follow-up flexible cystoscopy at 1 year post-resection showed no evidence of disease recurrence.
Bladder paraganglioma is rare and accounts for less than 1% of all catecholamine secreting neoplasms and only 0.5% of all bladder tumours[3]. They are thought to arise from embryonic rests of chromaffin cells within the bladder wall and often occur in young women in their second to fourth decade of life[4]. Paragangliomas that secrete catecholamine may give rise to clinical presentation similar to a hyperfunctioning adrenal phaeochromocytoma. Episodic symptoms may occur in spells or paroxysms, and can be highly variable. The position of these tumours within the bladder results in characteristic symptom complex related to micturition or over-distension of the bladder with catecholamine release. The majority of patients will experience micturition related hypertension, cold sweats, palpitations, headaches, dizziness and sweating much like the Case 1 in our series[5]. Systemic catecholamine secretion occurs with increased bladder pressure after bladder contraction, triggering these sympathomimetic attacks[6]. These symptoms may also be precipitated by defaecation, sexual activity, ejaculation or bladder instrumentation. Approximately 55%-60% of patients will also experience painless haematuria, although it is mostly microscopic in nature[7]. While hematuria is commonly reported in patient with bladder mass, this is not specific for paraganglioma. Only a minority of patients will experience weight loss, nausea, tremor, postural hypotension, syncope, chest pain, blurred vision, laryngismus, high blood sugar levels or symptoms associated with catecholamine cardiomyopathy[5].
Suspected cases of paragangliomas should first be investigated by measuring the level of catecholamine and its metabolites such as metanephrine and vanillylmandelic acid secretion in either the blood or urine. The measurement of serum adrenaline and noradrenaline can be costly and is usually unnecessary[8]. The majority of paragangliomas are not hormonally active, thus preoperative catecholamine levels maybe normal[1,8]. Bladder paraganglioma can often be difficult to distinguish radiologically from other bladder lesions[8]. The use of intravenous contrast may precipitates a hypertensive crisis although non-ionic contrast is reported to be safe[9]. In contrast to the hyperintense T2 signalling on magnetic resonance imaging with adrenal phaeochromocytoma, paraganglioma is likely to be homogenous on T2 signal[10]. Both I-131 MIBG and 18F-fluorodeoxyglucose, positron emission tomography are useful for localization of potential metastatic disease. I-123 MIBG can be used as an alternative to I-131 MIBG for accurate preoperative localization of small lymph node[11].
As many as half of the cases of paraganglioma share a genetic basisa and can be related to a number of hereditary conditions including VHL, neurofibromatosis type 1, Carney triad, Multiple Endocrine Neoplasia types 2a and 2b and familial paraganglioma[1]. Therefore, genetic testing should be offered in cases of young patient (under 50 years old) with a positive family history or with history of bilateral, extra-adrenal or multifocal phaeochromocytoma, a positive genetic mutation as well as those tumours with negative SDHB staining[12].
Since paraganglia are distributed throughout the bladder wall, paraganglioma can be found in any part of the bladder. These tumours are mostly well circumscribed and usually form nodules or small mass. Placing the tumour in a Zenker’s fixative will turn the tumour black; a positive chromaffin reaction. Histologically, paraganglioma is often misdiagnosed as urothelial carcinoma[13] and paraganglioma may mimic high grade urothelial carcinoma with a nest pattern. Features of paraganglioma include zellballen architecture where tumour cells are arranged into nests and lobules, a delicate fibrovascular stroma and eosinophilic cytoplasm. Immunohistochemistry is usually positive for NSE, chromogranin and synaptophysin and negative for cytokeratin. There are no definitive characteristics which reliably distinguish benign from malignant tumour, and desmoplastic reaction is often absent[12]. The distinction between benign and malignant paraganglioma has long been contentious, with the only widely accepted and definitive proof of malignancy being metastasis to other organs. Some histological features such as tumour necrosis, a mitotic rate greater than 3/30 high power field, capsular invasion, large nests with central degeneration, a lack of hyaline globules, a high nuclear/cytoplasmic ration, monotony of a cytological pattern and spindle cells patters are suggestive of increased malignant predilection[14]. Immunohistochemistry stains are often useful in helping to establish the diagnosis and those with SDHB negative-stain may indicate a succinate dehydrogenase subunit-mutated tumour. Urothelial carcinoma and carcinoid tumours are positive for cytokeratin, while melanoma cells show positivity for S100, HMB45 and Melan A stains.
Complete surgical removal of the tumour is the treatment of choice[1,4,6]. If the paraganglioma is a catecholamine secreting tumour, the effects of excess circulating catecholamines should be reversed prior to surgical extirpation. Combined preoperative α- and β-adrenergic blockade are required to control the blood pressure in order to prevent intra-operative hypertensive crisis. An α-adrenergic blockade should be commenced prior to β-adrenergic blockade, to allow for volume expansion of the contracted blood volume and a liberal salt diet and adequate hydration are also advised. Once adequate α-adrenergic blockade is achieved, β-adrenergic blockade can be initiated. Localised tumours can be removed in partial cystectomy while transurethral resection is adequate in superficial and small bladder lesion[8]. Postoperative 24 h urinary catecholamine and its metabolites should be conducted at week 2 and if the levels are normal, the resection of paraganglioma is considered complete.
As discussed earlier, malignant phaeochromocytoma remains a challenging entity to diagnose and treat. Up to 15% of bladder paraganglioma can become metastasis, and metastasis is the only reliable indicator of malignancy. Young age, extensive local disease and micturition attacks are risk factors for malignancy while features such as vascular invasion, a deeply invasive growth patterns and recurrence are often poor prognostic signs. Metastatic potential is often unclear and thus long-term annual follow up is suggested[15]. In patients with metastatic disease, complete cystectomy and pelvic lymph node dissection is the preferred option[16]. Nuclear imaging such as I-131 MIBG radiotherapy has also been shown to be useful for palliative control of tumour function in metastatic disease, but the current chemotherapy and radiotherapy treatment options are limited[17].
Bladder paraganglioma is a rare condition and patients can present with various clinical presentations. Biochemical profiling and nuclear imaging study can assist in the identification of this lesion. Preoperative care with volume hydration and adrenergic blockade are often necessary to control the blood pressure and to prevent intra-operative hypertensive crisis. Surgical extirpation remains the only cure for these patients and further research into this rare condition is warranted.
All patients presented with bladder mass with various clinical symptoms.
Bladder paraganglioma is diagnosed through biochemical hormonal profiling and nuclear imaging study.
Urothelial cancer, benign bladder lesion.
Measurement of catecholamine and its metabolites levels such as metanephrine and vanillylmandelic acid secretion in either the blood or urine.
Nuclear imaging study using iodine-131-meta-iodobenzylguanidine and 18F-fluorodeoxyglucose, positron emission tomography.
Features of paraganglioma include zellballen architecture where tumour cells are arranged into nests and lobules, a delicate fibrovascular stroma and eosinophilic cytoplasm. Immunohistochemistry is usually positive for neuron-specific enolase, chromogranin and synaptophysin and negative for cytokeratin.
Preoperative care with volume hydration and adrenergic blockade are often necessary to control the blood pressure and to prevent intra-operative hypertensive crisis. Surgical extirpation remains the only cure.
Bladder paraganglioma is a rare condition and patients can present with various clinical presentations. Malignant phaeochromocytoma remains a challenging entity to diagnose and treat, and further research into this rare condition is warranted.
This case series highlights the various clinical presentation of bladder paraganglioma and provides a clinical review of the current literature on management of this rare condition.
This is a well-written report of three cases of bladder paraganglioma. Bladder paraganglioma is very rare. Their pathological diagnosis is quite proper and clinical practice is also well-summarized.
P- Reviewer: El-Ghar MA, Kai K S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Young WF. Paragangliomas: clinical overview. Ann N Y Acad Sci. 2006;1073:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol. 1992;147:1-10. [PubMed] |
| 3. | Siatelis A, Konstantinidis C, Volanis D, Leontara V, Thoma-Tsagli E, Delakas D. Pheochromocytoma of the urinary bladder: report of 2 cases and review of literature. Minerva Urol Nefrol. 2008;60:137-140. [PubMed] |
| 4. | Cheng L, Leibovich BC, Cheville JC, Ramnani DM, Sebo TJ, Neumann RM, Nascimento AG, Zincke H, Bostwick DG. Paraganglioma of the urinary bladder: can biologic potential be predicted? Cancer. 2000;88:844-852. [PubMed] |
| 5. | Deng JH, Li HZ, Zhang YS, Liu GH. Functional paragangliomas of the urinary bladder: a report of 9 cases. Chin J Cancer. 2010;29:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Pastor-Guzmán JM, López-García S, Giménez-Bachs JM, Ruíz-Mondejar R, Cañamares-Pabolaza L, Atiénzar-Tobarra M, Casado-Moragón L, Virseda-Rodriguez JA. Paraganglioma of the bladder: controversy regarding treatment. Urol Int. 2004;73:270-275. [PubMed] |
| 7. | Tsai CC, Wu WJ, Chueh KS, Li WM, Huang CH, Wu CC, Lee MH, Chen SM. Paraganglioma of the urinary bladder first presented by bladder bloody tamponade: two case reports and review of the literatures. Kaohsiung J Med Sci. 2011;27:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Bhalani SM, Casalino DD, Manvar AM. Paraganglioma of the bladder. J Urol. 2011;186:279-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Bessell-Browne R, O’Malley ME. CT of pheochromocytoma and paraganglioma: risk of adverse events with i.v. administration of nonionic contrast material. AJR Am J Roentgenol. 2007;188:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Qiao HS, Feng XL, Yong L, Yong Z, Lian ZJ, Ling LB. The MRI of extraadrenal pheochromocytoma in the abdominal cavity. Eur J Radiol. 2007;62:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Furuta N, Kiyota H, Yoshigoe F, Hasegawa N, Ohishi Y. Diagnosis of pheochromocytoma using [123I]-compared with [131I]-metaiodobenzylguanidine scintigraphy. Int J Urol. 1999;6:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Huang KH, Chung SD, Chen SC, Chueh SC, Pu YS, Lai MK, Lin WC. Clinical and pathological data of 10 malignant pheochromocytomas: long-term follow up in a single institute. Int J Urol. 2007;14:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Zhou M, Epstein JI, Young RH. Paraganglioma of the urinary bladder: a lesion that may be misdiagnosed as urothelial carcinoma in transurethral resection specimens. Am J Surg Pathol. 2004;28:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, Giordano TJ, Greene LA, Goldstein DS, Lehnert H. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004;11:423-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 231] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Kappers MH, van den Meiracker AH, Alwani RA, Kats E, Baggen MG. Paraganglioma of the urinary bladder. Neth J Med. 2008;66:163-165. [PubMed] |
| 16. | Ansari MS, Goel A, Goel S, Durairajan LN, Seth A. Malignant paraganglioma of the urinary bladder. A case report. Int Urol Nephrol. 2001;33:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Gedik GK, Hoefnagel CA, Bais E, Olmos RA. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2008;35:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |