Published online Oct 16, 2014. doi: 10.12998/wjcc.v2.i10.578
Revised: June 23, 2014
Accepted: July 27, 2014
Published online: October 16, 2014
Processing time: 194 Days and 4.4 Hours
Introduction: World Health Organization announced on April 2009 a public health emergency of international concern caused by swine-origin influenza A (H1N1) virus. Acute respiratory distress syndrome (ARDS) has been reported to be the most devastating complications of this pathogen. Extracorporeal membrane oxygenator (ECMO) therapy for patients with H1N1 related ARDS has been described once all other therapeutic options have been exhausted. Here, we report the case of a child (German, male) with H1N1-associated fulminate respiratory and secondary hemodynamic deterioration who was rescued by initial emergent ECMO established through a dialysis catheter and subsequent switch to central cannulation following median sternotomy. This report highlights several important issues. First, it describes a successful use of a dialysis catheter for the establishment of a veno-venous ECMO in an emergency case by child. Second, it highlights the importance of a closely monitoring of clotting parameters during ECMO therapy and third, if severe respiratory failure is complicated by cardiogenic shock, veno-atrial ECMO support via median sternotomy should be considered as a viable treatment option without further delay.
Core tip: Here, we report the case of a child with swine-origin influenza A-associated fulminate respiratory and secondary hemodynamic deterioration, who was rescued by initial emergent extracorporeal membrane oxygenator (ECMO) established through a dialysis catheter and subsequent switch to veno-atrial ECMO (VA-ECMO) via central cannulation. This report highlights several important issues. First, it describes a successful use of a dialysis catheter for the veno-venous ECMO-establishment in an emergency case by child. Second, it highlights the importance of a closely monitoring of clotting parameters and third, if severe respiratory failure is complicated by cardiogenic shock, VA-ECMO support via median sternotomy should be considered as a viable treatment option without further delay.
- Citation: Papadopoulos N, Martens S, Keller H, El-Sayed Ahmad A, Moritz A, Zierer A. Challenging rescue of a 4 years old boy with H1N1 infection by extracorporeal membrane oxygenator: A case report. World J Clin Cases 2014; 2(10): 578-580
- URL: https://www.wjgnet.com/2307-8960/full/v2/i10/578.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i10.578
Establishment of extracorporal membrane oxygenation (ECMO) through percutaneous placement of cannulas in children can be difficult because of the small vessel size[1]. Median sternotomy may be necessary in selected cases to cannulate the ascending aorta and right atrium for sufficient ECMO flow[1,2].
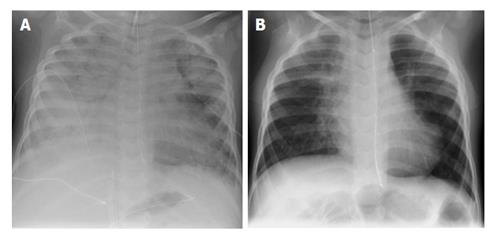
A 4 years old German boy presented in our emergency room with a 24 h history of shortness of breathe following several days of an influenza-like illness. At presentation, he suffered from respiratory failure requiring urgent intubation and mechanical ventilation. After his admission to our intensive care unit the initial chest radiograph revealed bilateral patchy infiltrates (Figure 1A). H1N1 influenza virus was confirmed by the reverse transcriptase-polymerase chain reaction assay of respiratory secretions. Bacterial cultures were negative. He was treated empirically with Oseltamivir. Within 24 h after hospital admission the patient had severely impaired gas exchange despite maximum respiratory support on the ventilator. For this reason high frequency oscillation ventilation (HFOV) was initiated. Since there was no improvement of the respiratory situation within 4 d of HFOV-treatment, veno-venous ECMO (VV-ECMO) had to be established.
Because of the small vessel size of the child, save cannulation sites for percutaneous placement of the ECMO cannulas were limited and included the internal jugular veins and the femoral veins. Placement of a cannula through the jugular vein was not successful. The child was in critical clinical condition and we decided to start VV-ECMO support via an 11 Fr dialysis catheter (Dolphin Protect, Gambo, Hechingen, Germany) placed into the left femoral vein. We established ECMO outflow of oxygenated blood via the arterial lumen and inflow of deoxygenated blood via the venous lumen of the dialysis catheter. We connected ECMO-tubes with the dialysis catheter via a connector (1/4 × LLm, Maquet GETINGE GROUP, Hirrlingen, Germany). ECMO circuit consisted of a Quadrox id pediatric (Maquet Cardiovascular, Wayne, NJ, United States) polymethylpentene oxygenator and a Rotaflow (Maquet Cardiovascular) centrifugal pump. This setting allowed for a flow of 400-milliliter per min. Despite the rather low circuit flow, the combination of VV-ECMO and HFOV allowed for an immediate improvement of oxygenation and weaning of vasopressor support.
Three days later, following an initial course of stabilization a sudden exchange of the VV-ECMO-system had to be performed due to massive clot-formations in the oxygenator. Notably there was an exponential elevation of fibrinogen and D-Dimer, as a result of disseminated intravascular coagulation (DIC), which could be detected from the establishment of the VV-ECMO support on until the system change. In order to avoid further clotting formations continuous application of heparin directly in the venous cannula of the new ECMO circuit has been established.
One day after the system change a sudden hemodynamic instability required high inotropic and vasopressor support. Therefore we decided to switch the VV-ECMO to veno-atrial ECMO (VA-ECMO). In order to reliably maintain adequate flow in this critical situation we performed a median sternotomy and established VA-ECMO support via the right atrial appendage and the ascending aorta. A Bio-Medicus (Medtronic, Inc., Minneapolis, MI, United States) arteria cannula was used as the return cannula for oxygenated blood. For venous drainage a multi-port Bio-Medicus (Medtronic, Inc.) cannula was used. Circuit flow of 1.2 liters min-1 led to a stabilization of the hemodynamic situation with immediate weaning from the vasopressor and intotropic support.
VA-ECMO was provided for a total of 10 d and could afterwards be successfully explanted. The patient could be successfully decannulated and control chest radiograph showed normal lung morphology (Figure 1B). The 4-year-old boy could be discharged from the hospital after a total of 38 d with full resolution of symptoms.
This report highlights several important issues. First, it describes a successful use of a dialysis catheter for the establishment of a VV-ECMO in an emergency case, in which, due to the small vessel size of the child, the percutaneous placement of routine ECMO cannulas was not possible. Second, as clotting formations in the ECMO-oxygenator is a possible and devastating complication especially in critically ill patients with H1N1 infection suffering a DIC, it is vital that clotting parameters, especially fibrinogen and D-Dimer, of such patients are closely monitored. Third, if severe respiratory failure is complicated by cardiogenic shock, VA-ECMO support via median sternotomy should be considered as a viable treatment option without further delay[3-9].
The 4 years old patient presented in the emergency room with the main symptom of dyspnoea.
Clinical diagnosis of acute respiratory failure leads to an urgent intubation and mechanical ventilation of the young boy.
Bacterial infection could be excluded once the bacterial cultures were negative.
Swine-origin influenza A (H1N1) influenza virus was confirmed by the reverse transcriptase-polymerase chain reaction assay of respiratory secretions.
After his admission to the authors intensive care unit the initial chest radiograph revealed bilateral patchy infiltrates.
Within 24 h after hospital admission the patient had severely impaired gas exchange despite maximum respiratory support on the ventilator.
For this reason high frequency oscillation ventilation (HFOV) was initiated. Due to the fulminate respiratory and secondary hemodynamic deterioration, initial emergent veno-venous extracorporeal membrane oxygenator (VV-ECMO) (extracorporeal membrane oxygenator) established through a dialysis catheter and subsequent switches to veno-atrial ECMO (VA-ECMO) through central cannulation following median sternotomy, has to be performed.
ECMO therapy for patients with H1N1 related acute respiratory distress syndrome (ARDS) has been described once all other therapeutic options have been exhausted. This report highlights several important issues. First, it describes a successful use of a dialysis catheter for the establishment of a VV-ECMO in an emergency case by child. Second, it highlights the importance of a closely monitoring of clotting parameters during ECMO therapy and third, if severe respiratory failure is complicated by cardiogenic shock, VA-ECMO support via median sternotomy should be considered as a viable treatment option without further delay.
This manuscript lights on the problem of the ECMO cannulation in emergency and in the pediatric patient, and indicate as a solution the use of the dialysis catheter instead of the double lumen pediatric ECMO’s cannula.
P- Reviewer: Belliato M, Guo YZ, Pocar M S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Shuhaiber J, Thiagarajan RR, Laussen PC, Fynn-Thompson F, del Nido P, Pigula F. Survival of children requiring repeat extracorporeal membrane oxygenation after congenital heart surgery. Ann Thorac Surg. 2011;91:1949-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Alsoufi B, Al-Radi OO, Gruenwald C, Lean L, Williams WG, McCrindle BW, Caldarone CA, Van Arsdell GS. Extra-corporeal life support following cardiac surgery in children: analysis of risk factors and survival in a single institution. Eur J Cardiothorac Surg. 2009;35:1004-1011; discussion 1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec. 2009;84:249-257. [PubMed] |
| 4. | Novel influenza A(H1N1) investigation team. Description of the early stage of pandemic (H1N1) 2009 in Germany, 27 April-16 June 2009. Euro Surveill. 2009;14:pii: 19295. [PubMed] |
| 5. | Poggensee G, Gilsdorf A, Buda S, Eckmanns T, Claus H, Altmann D, Krause G, Haas W. The first wave of pandemic influenza (H1N1) 2009 in Germany: from initiation to acceleration. BMC Infect Dis. 2010;10:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Sihler KC, Park PK. Extracorporeal membrane oxygenation in the context of the 2009 H1N1 influenza A pandemic. Surg Infect (Larchmt). 2011;12:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Papadopoulos N, Ahmad Ael-S, Marinos S, Moritz A, Zierer A. Extracorporeal membrane oxygenation for influenza-associated acute respiratory distress syndrome. Thorac Cardiovasc Surg. 2013;61:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Extracorporeal Life Support Organization (ELSO). H1N1 Specific Supplements to the ELSO General Guidelines (Extracorporeal Life Support Organization). 2009;1-4. |
| 9. | Riscili BP, Anderson TB, Prescott HC, Exline MC, Sopirala MM, Phillips GS, Ali NA. An assessment of H1N1 influenza-associated acute respiratory distress syndrome severity after adjustment for treatment characteristics. PLoS One. 2011;6:e18166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |