Published online Oct 16, 2014. doi: 10.12998/wjcc.v2.i10.565
Revised: June 23, 2014
Accepted: July 18, 2014
Published online: October 16, 2014
Processing time: 145 Days and 2.3 Hours
Chemotherapy has been linked with Takotsubo cardiomyopathy. Most of the literature on chemotherapy associated Takotsubo cardiomyopathy is on the drug 5-fluorouracil. In this report, we describe the case of a 55-year-old Asian male who developed Takotsubo cardiomyopathy while receiving dual chemotherapy with cytarabine and daunorubicin for acute myeloid leukemia. To our knowledge, it is the first case of Takotsubo cardiomyopathy associated with daunorubicin and/or cytarabine.
Core tip: In this case report, we describe first case of Takotsubo cardiomyopathy associated with daunorubicin and/or cytarabine.
- Citation: Goel S, Sharma A, Garg A, Chandra A, Shetty V. Chemotherapy induced Takotsubo cardiomyopathy. World J Clin Cases 2014; 2(10): 565-568
- URL: https://www.wjgnet.com/2307-8960/full/v2/i10/565.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i10.565
Chemotherapeutic drugs have a wide range of cardio-toxic effects. Recently, there have been case reports of chemotherapy [namely 5-fluorouracil (5-FU)] induced Takotsubo cardiomyopathy (TC)[1-5]. However, to the best of our knowledge, there has been no published literature on cytarabine and/or daunorubicin causing TC. In this report, we describe the case of a 55-year-old Chinese male who developed TC while receiving dual chemotherapy with cytarabine and daunorubicin for non M3 acute myeloid leukemia.
A 55-year-old male with past medical history of diabetes mellitus (type II) presented to our hospital with complaints of pleuritic chest pain with non-productive cough and fever (Tmax 101.2 °F) for 3 d. Chest X-ray showed right-sided lung infiltrates. Patient was admitted to the medical floor with the diagnosis of community-acquired pneumonia and was started on moxifloxacin. The patient’s blood work showed an incidental finding of 12% blast cells with a total white cell count of 9.9. Electrocardiogram performed on the day of admission revealed sinus tachycardia with abnormal R wave progression. Echocardiogram showed ejection fraction (EF) of 60%-65%, with normal chamber size and mild diastolic dysfunction. Three sets of cardiac enzymes including cardiac Troponin I and creatine kinase-MB were negative. Patient was evaluated by the hematology and oncology team for the incidental finding of blast cells on peripheral blood smear. The next day, as per the hematologist’s recommendation, the patient underwent a bone marrow biopsy which showed the presence of pro-myelocytes, suggestive of M3 acute myeloid leukemia. The patient was started on All Trans-Retinoic Acid induction chemotherapy regimen. Prophylactic valacyclovir, omeprazole and intravenous (iv) fluids were also started.
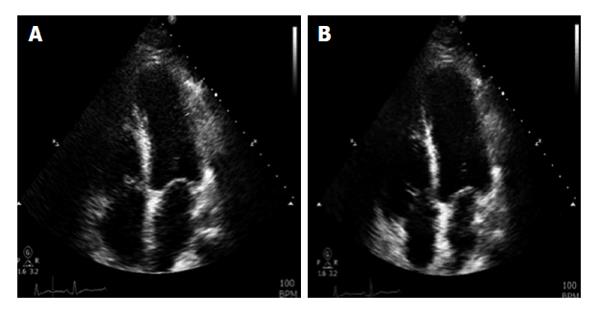
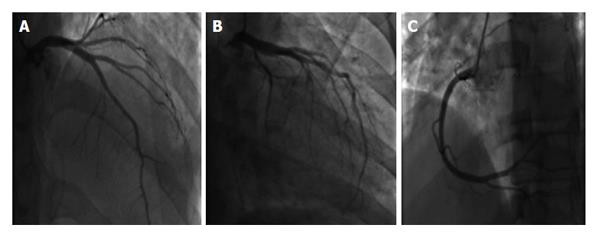
Two days after the initiation of chemotherapy, fluorescent in situ hybridization results demonstrated a negative translocation of chromosomes 15, 17, thus confirming the diagnosis of non-M3. As a result, the chemotherapy regimen was changed to cytarabine 100 mg/m2 and daunorubicin 60 mg/m2. On day 6 of chemotherapy with cytarabine and daunorubicin, the patient began to experience non-radiating sub sternal chest pain associated with palpitations. Electrocardiogram obtained at that time showed sinus tachycardia of 170 bpm with ST segment elevation in leads I, aVL, V5, V6; consistent with anterolateral wall ST elevation myocardial infarction (STEMI). The patient was transferred to the cardiac intensive care unit (CCU) with a diagnosis of STEMI. Cardiac enzymes were obtained which showed Cardiac Troponin I of 8.54 upon initial transfer to the CCU, reaching a maximum of 38.64 after 18 h (normal values 0-0.1 ng/mL). Given the patient’s immunocompromised state, cardiac catheterization was deferred and he was managed medically with aspirin, clopidogrel, rosuvastatin, and aggressive iv hydration. Echocardiogram done on day 6 of chemotherapy showed an EF of 30%-35% with segmental wall motion abnormalities: mild anterior, septal, apical, inferior and lateral wall hypokinesia, with normal diastolic function consistent with mid-left anterior descending artery occlusion (Figure 1). On day 20 of admission, patient underwent an elective cardiac angiogram, which showed non-obstructive coronary vasculature, mildly decreased left ventricular systolic function, EF of 50% with mild anterolateral and anterobasal hypokinesia (Figure 2).
This case report demonstrates a strong causal relationship between chemotherapy and the development of TC as evidenced in the patient’s presentation on day 6 of chemotherapy induction. Symptomatic recovery of the patient after supportive medical management, with the concomitant discontinuation of the chemotherapeutic agent, also strengthens this causal relationship. The patient’s repeat echocardiogram (performed 2 wk after discontinuation of the chemotherapeutic agents) showed a complete recovery of the EF with no wall motion abnormalities. In addition, a coronary angiogram demonstrated non-obstructed coronary vasculature. Given the patient’s clinical presentation and the diagnostic evidence obtained, there is no alternative justification for the clinical course observed other than Takotsubo cardiomyopathy. This is the first case report of daunorubicin and/or cytarabine induced TC.
Most of the literature on chemotherapy associated TC is published on the drug 5-FU, a widely used chemotherapeutic agent for solid tumors. One case report from Japan described daunorubicin-induced TC in a patient with refractory multiple myeloma[6]. However, to our knowledge, this is the first case report of daunorubicin and/or cytarabine induced TC in the United States.
Chemotherapy induces increased sympathetic tone with resulting elevation of cytokine, free radical, prostaglandin, catecholamine and growth factor levels. The excess of these modulators can potentiate worsening adrenoreceptor sensitivity, and can contribute to the clinical presentation of TC [1-5]. Daunorubicin belongs to the anthracycline class of chemotherapeutic agents, which remains among the most active anti-cancer drugs for solid tumor and hematologic malignancies. The exact pathogenic mechanisms responsible for the underlying cardio-toxic effects of anthracycline agents has yet to be elucidated. The current postulated mechanism supports the role of free radical induced cardiac damage (known to be caused by the excessive production of hydrogen peroxide, hydroxyl radicals and reactive oxygen species)[6-10]. These free radicals promote lipid peroxidation which contributes to cell membrane damage, and thus results in the activation of pro-apoptotic enzymes, such as Bax, Cytochrome-c and caspase-3, in myocyte mitochondria, triggering apoptosis and resulting in cardiac myocyte cell death[6-10]. Cardiac myocytes are more susceptible to lipid peroxidation due the presence of a high mitochondrial density with resultant high-energy requirements and the lack of anti-oxidant enzymes, which are required for the detoxification of superoxide anions and hydrogen peroxide. As a result of this cardiac myocyte susceptibility, a dose-related and irreversible loss of cardiac myocytes occurs, resulting in cardiomyopathy[11]. Though the exact mechanism of cardiotoxicity caused by cytarabine has yet to be elucidated, it is postulated that this drug can result in a hypersensitivity reaction or possible immune-mediated damage of cardiac myocyte[12].
In conclusion, we suggest that physicians be vigilant when treating patients with daunorubicin and/or cytarabine and should be aware of a possible association of these chemotherapeutic agents with TC.
A 55-year-old male receiving treatment with Daunorubicin and Cytarabine for non M3 acute myeloid leukemia (AML) experience non-radiating sub sternal chest pain associated with palpitations on 6th day after chemotherapy.
Acute coronary syndrome.
ST segment elevation myocardial infarction (STEMI), non-STEMI, Unstable Angina, Aortic Dissection, Pulmonary embolism, cardiomyopathy, Ventricular wall rupture.
Cardiac troponin I and creatine kinase-MB elevation with continued uptrend, consistent with myocardial ischemia.
Electrocardiogram-anterolateral STEMI; Echocardiogram (ECHO) at day 6 of therapy-ejection fraction (EF) of 30%-35% with segmental wall motion abnormalities; repeat ECHO (two weeks later)-Normalization of EF and no wall motion abnormalities; cardiac catheterization (two weeks later)-clean coronaries. Pt diagnosed with Takotsubo cardiomyopathy.
Due to patient’s immunocompromised state, he was medically managed with aspirin, clopidogrel, rosuvastatin, and aggressive intravenous hydration in cardiac intensive care unit.
Patient initially thought to have acute coronary syndrome but was eventually found to have Takotsubo cardiomyopathy. Patient had clean coronaries on Cardiac catheterization and on repeat ECHO, his EF normalized and wall motion abnormalities resolved.
Non M3 AML-According to French-American-British classification acute myeloid leukemia are sub grouped in to 8 categories M0-M7. This classification guides the therapy and prognosis in patients diagnosed with AML.
Vigilance should be observed while treating patients with daunorubicin and/or cytarabine.
This is first case report of takotsubo cardiomyopathy associated with daunorubicin and/or cytarabine.
P- Reviewer: de Botton S, Kurpisz MK, Tobita K S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
| 1. | Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 505] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 2. | Smith SA, Auseon AJ. Chemotherapy-induced takotsubo cardiomyopathy. Heart Fail Clin. 2013;9:233-242, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Y-Hassan S, Tornvall P, Törnerud M, Henareh L. Capecitabine caused cardiogenic shock through induction of global Takotsubo syndrome. Cardiovasc Revasc Med. 2013;14:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Grunwald MR, Howie L, Diaz LA. Takotsubo cardiomyopathy and Fluorouracil: case report and review of the literature. J Clin Oncol. 2012;30:e11-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Stewart T, Pavlakis N, Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J. 2010;40:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Mitsumori T, Nakajima K, Nozaki Y, Hamanaka S, Nagashima T, Kirito K, Iwao N, Komatsu N. [Multiple myeloma complicated with Takotsubo cardiomyopathy]. Rinsho Ketsueki. 2010;51:291-296. [PubMed] |
| 7. | Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1636] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 8. | Chua CC, Liu X, Gao J, Hamdy RC, Chua BH. Multiple actions of pifithrin-alpha on doxorubicin-induced apoptosis in rat myoblastic H9c2 cells. Am J Physiol Heart Circ Physiol. 2006;290:H2606-H2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62:4592-4598. [PubMed] |
| 10. | Wang GW, Klein JB, Kang YJ. Metallothionein inhibits doxorubicin-induced mitochondrial cytochrome c release and caspase-3 activation in cardiomyocytes. J Pharmacol Exp Ther. 2001;298:461-468. [PubMed] |
| 11. | Goormaghtigh E, Huart P, Praet M, Brasseur R, Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem. 1990;35:247-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Williams SF, Larson RA. Hypersensitivity reaction to high-dose cytarabine. Br J Haematol. 1989;73:274-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |