Published online Oct 16, 2014. doi: 10.12998/wjcc.v2.i10.522
Revised: July 7, 2014
Accepted: July 25, 2014
Published online: October 16, 2014
Processing time: 167 Days and 1.2 Hours
Endoscopic retrograde cholangiopancreatography (ERCP) is a procedure that can result in serious complications, and thus should be handled by a skilled endoscopist to minimize the risk of complications and to enhance the success rate. The incidence of ERCP-related complications is 5%-10%, most commonly involving post-ERCP pancreatitis and clinically significant post-endoscopic sphincterotomy bleeding. Although ERCP-related perforation has a relatively lower incidence of 0.14%-1.6%, this complication is associated with a high mortality rate of 4.2%-29.6%. A classification of perforation type based on the instrument that caused the perforation was recently described that we postulated could affect the implementation of perforation management. In the present article, an algorithm for management and prevention of ERCP-related perforations is proposed that is based on the perforation type and delay of diagnosis. Available evidence demonstrates that a delayed diagnosis and/or treatment of perforation results in a poorer prognosis, and thus should be at the forefront of procedural consideration. Furthermore, this review provides steps and recommendations from the pre-procedural stage through the post-procedural evaluation with consideration of contributing factors in order to minimize ERCP-related complication risk and improve patient outcome. To avoid perforation, endoscopists must evaluate the risks related to the individual patient and the procedure and perform the procedure gently. Once a perforation occurs, immediate diagnosis and early management are key factors to minimize mortality.
Core tip: Endoscopic retrograde cholangiopancreatography (ERCP)-related perforation, is a rare complication with a high morbidity and mortality. An immediate diagnosis and early management of ERCP-related perforation are key factors to minimize mortality. In this review article, the authors shared their experiences and propose an algorithm to avoid perforation and for management once a perforation occurs.
- Citation: Prachayakul V, Aswakul P. Endoscopic retrograde cholangiopancreatography-related perforation: Management and prevention. World J Clin Cases 2014; 2(10): 522-527
- URL: https://www.wjgnet.com/2307-8960/full/v2/i10/522.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i10.522
Endoscopic retrograde cholangiopancreatography (ERCP) is a procedure that should be performed by a skilled endoscopist to maximize the success rate and minimize complications, which occur in 5%-10% of cases[1,2]. The most common complications are post-ERCP pancreatitis (1.0%-3.5% of cases)[3-5] and clinically significant post-endoscopic sphincterotomy bleeding (0.1%-2.0% incidence)[6-9]. ERCP-related perforations are relatively uncommon (incidence of 0.14%-1.6%), though associated with a high mortality rate of 4.2%-29.6%[10,11]. Whereas delayed recognition and treatment of this complication contribute to fatality, early detection and management confer a better prognosis. This review focuses on the classification, early diagnosis, management, and prevention of ERCP-related perforations.
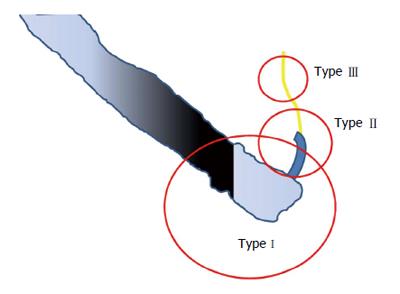
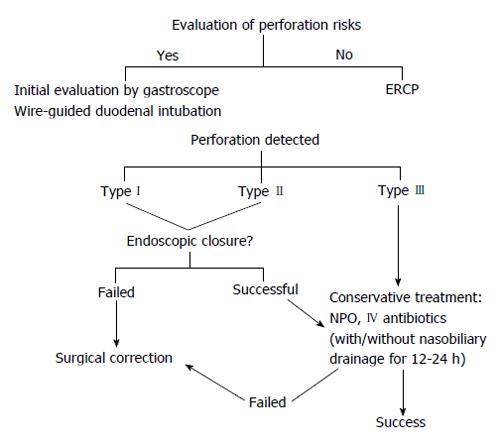
ERCP-related perforations were previously classified into 3-4 types, regardless of the site of perforation[12,13] (Table 1). In 2011, Kim et al[14] proposed a new classification based on the instrument that caused the perforation. A type I perforation results from the scope itself, which always causes a large perforation with heavy contamination (Figure 1). A type II perforation can be caused by the needle-knife used during the sphincterotomy, from the ERCP cannula or the sphincterotome, which cause a moderate-sized hole with less contamination. Type III refers to a perforation caused by the guidewire and is associated with the least risk of contamination. A review of cases presented by Kim et al[14] and an additional 62 cases from Kwon et al[15] that were reevaluated using the new classification, revealed that 80% of patients with type I perforations (20/25 cases) required surgery, compared to 19% (7/37) of those with type II perforations. We therefore postulated that this classification could be used to direct the management of patients with perforations.
| According to Stapfer et al[12] | According to Howard et al[13] | |
| Type I | Lateral or medial wall perforation | Duodenal perforation remote from the papilla |
| Type II | Perivaterian injury | Periampullary retroperitoneal perforation |
| Type III | Distal bile duct injury related to wire/basket instrumentation | Guidewire perforation |
| Type IV | Retroperitoneal air alone | None |
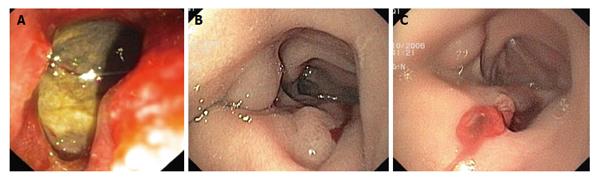
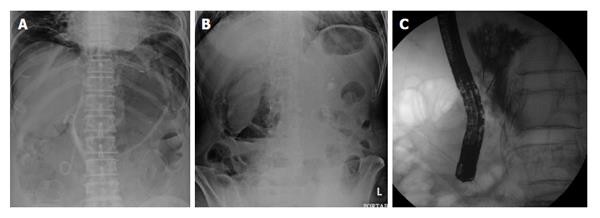
Most type I perforations are immediately recognized by the endoscopist during the procedure. Large perforations can be definitively indicated by visualization of intra-abdominal organs, whereas smaller perforations may show only yellowish tissue of intra/retroperitoneal fat or by bleeding from other sites such as a lateral wall of the duodenum (Figure 2). If the endoscopist who performed the procedure suspects a perforation, evaluation of the pneumoperitoneum by fluoroscopy can be very helpful (Figure 3A). Some experts also recommend changing the duodenoscope to an end-view type for better mucosal visualization.
Type IIs are typically retroperitoneal perforations that occur during treatment interventions. In some cases, the endoscopist may recognize that an injury is “too deep” with increased bleeding, abnormal positioning of the guidewire, skin emphysema, and a clear kidney shadow. When this type of perforation is suspected, it should be confirmed by fluoroscopy, which can help identify unexplainable air in the retroperitoneum (Figure 3B), or by contrast injection, which will show contrast leakage into the retroperitoneal cavity (Figure 3C).
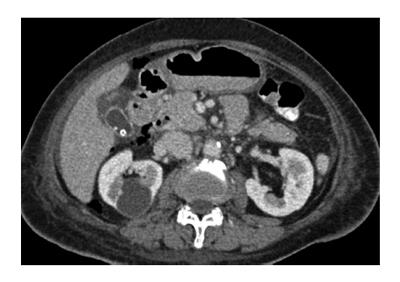
Type III perforationscan be recognized by an unusual guidewire position. If recognized in a timely manner, this type of perforation can be adequately managed by simply pulling the guidewire back into a safe position. However, the perforation can become a type II if the endoscopist does not recognize the perforation and continues pushing the instrument further. Up to 20%-30% of type II and III perforations are not immediately diagnosed, and patients may report abdominal pain and discomfort, which are followed by fever and leukocytosis. These perforations can be confirmed by a computed scan of the abdomen, which reveals retroperitoneal air (Figure 4) or fluid collection[16].
General principles of management for ERCP-related perforation include a nil per os directive, iv fluid resuscitation, administration of antibiotics to decrease intra/retroperitoneal contamination or fluid collection, pneumoperitoneal decompression, and, if possible, endoscopic closure. In some cases, surgical consultation may also be needed to control sepsis and repair the perforation, depending on the site and degree of leakage, the patient’s condition, and mechanism of injury. Radiologic interventions were found to be useful in some particular cases, particularly those with localized retroperitoneal fluid collection and without clinical sign of peritonitis[17]. However, the management of ERCP perforation in that particular study was based upon whether the perforation was diagnosed immediately after it occurred, or delayed by at least 24 h after the procedure (recognized by abnormal vital or abdominal signs).
Type I perforations, which are typically 1.0-1.3 cm wide, have been successfully treated during ERCP with hemostatic clips either through the gastroscope(with or without cap assistance)[18,19] or with an endoloop[20], or with over-the-scope clips[21]. Contrast injections should be administered to rule out leakage, as 30%-60% of type I perforations fail endoscopic closure and require surgery. Patients who undergo immediate surgical correction typically remain in the hospital for 12-16 d. Prompt diagnosis is crucial, as evidenced by a study of Miller et al[11] that reported two deaths out of five cases involving type I perforations, resulting from delayed diagnosis in one case, and delayed operation in the other, and six deaths from delayed surgical repair of type II perforations that were initially managed with a conservative treatment strategy[11]. Thus, some surgeons recommend immediate diagnosis and early surgery for ERCP-related perforations[22-24]. In our endoscopic center (within a tertiary care, university-based hospital), 4082 ERCP procedures were performed between January 2009 and June 2013, with a post-ERCP perforation rate of 0.29% (n = 10 type I; n =2 type II perforation cases). All of the patients (65-91 years old) were diagnosed during or immediately upon finishing the ERCP procedures. Eighty-three percent of these cases underwent surgical correction while only 17% received conservative treatment, with no deaths.
Mao et al[25] reported on nine cases of ERCP-related perforation (mostly type II), six of which were managed conservatively with hospital stays ranging from 4 to 75 d. Subcutaneous emphysema was a significant clinical sign found in seven of the nine patients. To aid in the success of conservative treatments, which included antibiotics, endoscopic treatment (when possible), intensive observation and follow-up imaging, nasobiliary and gastrointestinal drainage was recommended to reduce the leakage of digestive juices. However, very high success rates were also reported with conservative treatment (with or without nasobiliary drainage) by Park et al[26] and Vezakis et al[27], using fully covered self-expandable metal stents for closure of perforated sites at the ampullary region.
The majority of type III perforations involve only mild contrast leakage or pneumoperitoneum without contrast leakage, and can be managed conservatively, requiring hospitalization for only 5-6 d[11,24]. Genzlinger et al[28] found that up to 29% of patients who underwent ERCP showed pneumoperitoneum on plain radiography without clinical significance. However, further investigation is recommended in patients who have pneumoperitoneum and clinical signs of infection.
The surgical indications in the studies mentioned here differed from recommendations stated by Stapfer et al[12], which include large extravasation of contrast, fluid collection in the peritoneal or retroperitoneal space on follow-up computed tomography scan, massive subcutaneous emphysema, or retained choledocholithiasis. Our recommendation is to perform salvage surgery in patients with: (1) failure of initial endoscopic closure, especially type I or type II perforations; (2) no improvement in clinical signs of sepsis, or follow-up abdominal signs worsening within 12-24 h after successful endoscopic closure (type I) or conservative management (type II); and (3) retained instruments or choledocholithiasis requiring surgical removal.
The prognosis is generally worse for any type of perforation with a delayed diagnosis, and most patients develop clinical signs of peritonitis and sepsis within 1-5 d after the procedure[26-28]. It is important to note that abdominal signs might be relatively benign within the first few hours, even for type I perforations, however the presentation of peritoneal signs as a late finding is related to a poor clinical outcome[11]. Surgical drainage and correction is recommended in cases of delayed type II diagnosis with clinical sepsis[11,21,26,27]. However, most patients (50%-90%) having type II perforations with minimal leakage or type III perforations will respond to conservative treatment.
There are several factors that have been associated with an increased risk for ERCP-related perforation (Table 2). Nonetheless, there are steps that can be taken to avoid causing ERCP-related perforations, beginning before the procedure, and continuing through the follow-up stage (Figure 5).
| Factors | Results of multivariate analysis [OR (95%CI)] |
| Dilated common bile duct | 2.32 (1.02-5.03) |
| Sphincter of oddi dysfunction | 3.20 (1.64-8.94) |
| Longer duration of procedure | 1.02 (1.00-1.04) |
| Biliary stricture dilatation | 7.29 (1.84-28.11) |
| Performance of sphincterotomy | 6.94 (2.43-19.77) |
Some patients have a higher risk of perforation because of surgically altered anatomy. Such patients should undergo “endoscopic scanning” using an end-view gastroscope before starting ERCP. In cases of failure to pass the end-view scope owing to anastomotic stricture or too much resistance, continuation with the ERCP procedure should first be discussed with the patients and their families. If the endoscopist decides to follow through with the procedure, a duodenoscope should be inserted in an “over-the-guidewire” fashion to minimize duodenal wall injury. When narrowing of the anastomosis site is encountered, balloon dilatation over the guidewire can be helpful prior to duodenoscope insertion. However, this is not recommended for cases with malignant strictures, but rather duodenal stenting with a self-expandable metal stent followed by ERCP in the subsequent weeks is more appropriate.
There are several comments and recommendations concerning the procedure that should be kept in mind: (1) Patients with periampullary diverticula have a higher risk of perforation, especially from sphincterotomy. Therefore, the endoscopist must be very careful during this process. A balloon sphincteroplasty is recommended in such cases, rather than endoscopic sphincterotomy; (2) Insertion of any ERCP instruments into the bile duct should be performed in an “over-the-guidewire” fashion to prevent a “false track.” Furthermore, the endoscopist should perform ERCP as gently as possible to minimize tissue injury; (3) Endoscopic sphincterotomy should be carried out in the suggested direction of 11 to 12 o’clock for the common bile duct and 12 to 2 o’clock for pancreatic sphincterotomy. Over-bowing of the sphincterotome should be avoided to prevent a “zipper cut,” and cutting should not go beyond the second fold above the papilla. Adequate knowledge and intensive observation of ampulla anatomy are essential for all endoscopists who perform ERCP; (4) Needle-knife sphincterotomy should be performed only by experienced endoscopists. Those who are less experienced should recognize their limitations and be responsible enough to request assistance; and (5) Dilatation of the ampulla increases the risk of perforation. Therefore, the endoscopist should consider the length of sphincterotomy or the size of balloon sphincteroplasty suitable for the objective of the maneuver, such as limited or no sphincterotomy for stenting, dilating the ampulla no more than the size of the common bile duct above, and an appropriately sized sphincterotomy depending on the size of the stone.
The endoscopist should observe the patient’s abdominal signs and perform fluoroscopy intermittently during the ERCP procedure. A diagnosis can be immediately made upon recognition of an abnormal guidewire position, extravasation of contrast, or pneumoperitoneum, which can allow for prompt treatment. Furthermore, a fluoroscopic abdominal scan should be performed after any difficult procedure to identify possible complications.
Patients should be encouraged to promptly report any abdominal symptoms and should be watched for abnormal clinical signs after the ERCP procedure. The ward staff should be notified to maintain special observation of patients with difficult cases for early detection of possible complications.
As ERCP-related perforations are a serious complication with a high mortality rate, endoscopists should perform the procedure with caution. To avoid perforation, endoscopists must evaluate the risks related to the patient and the procedure and perform the procedure with care. Once a perforation occurs, immediate diagnosis and early management are key factors to minimize mortality.
P- Reviewer: Murata A, Thomopoulos KC S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Loperfido S, Angelini G, Benedetti G, Chilovi F, Costan F, De Berardinis F, De Bernardin M, Ederle A, Fina P, Fratton A. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998;48:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 779] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 2. | Wu HM, Dixon E, May GR, Sutherland FR. Management of perforation after endoscopic retrograde cholangiopancreatography (ERCP): a population-based review. HPB (Oxford). 2006;8:393-399. [PubMed] |
| 3. | Maranki J, Yeaton P. Prevention of post-ERCP pancreatitis. Curr Gastroenterol Rep. 2013;15:352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Masci E, Toti G, Mariani A, Curioni S, Lomazzi A, Dinelli M, Minoli G, Crosta C, Comin U, Fertitta A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol. 2001;96:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 613] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 5. | Jeurnink SM, Siersema PD, Steyerberg EW, Dees J, Poley JW, Haringsma J, Kuipers EJ. Predictors of complications after endoscopic retrograde cholangiopancreatography: a prognostic model for early discharge. Surg Endosc. 2011;25:2892-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zippi M, De Felici I, Pica R, Agus MA, Solinas A, Occhigrossi G, Traversa G. Self-expandable metal stent placement for treatment of severe sphincterotomy bleeding. Clin Ter. 2013;164:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Dunne R, McCarthy E, Joyce E, McEniff N, Guiney M, Ryan JM, Beddy P. Post-endoscopic biliary sphincterotomy bleeding: an interventional radiology approach. Acta Radiol. 2013;54:1159-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Balmadrid B, Kozarek R. Prevention and management of adverse events of endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am. 2013;23:385-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Yang XM, Hu B. Endoscopic sphincterotomy plus large-balloon dilation vs endoscopic sphincterotomy for choledocholithiasis: a meta-analysis. World J Gastroenterol. 2013;19:9453-9460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Alfieri S, Rosa F, Cina C, Tortorelli AP, Tringali A, Perri V, Bellantone C, Costamagna G, Doglietto GB. Management of duodeno-pancreato-biliary perforations after ERCP: outcomes from an Italian tertiary referral center. Surg Endosc. 2013;27:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Miller R, Zbar A, Klein Y, Buyeviz V, Melzer E, Mosenkis BN, Mavor E. Perforations following endoscopic retrograde cholangiopancreatography: a single institution experience and surgical recommendations. Am J Surg. 2013;206:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Stapfer M, Selby RR, Stain SC, Katkhouda N, Parekh D, Jabbour N, Garry D. Management of duodenal perforation after endoscopic retrograde cholangiopancreatography and sphincterotomy. Ann Surg. 2000;232:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Howard TJ, Tan T, Lehman GA, Sherman S, Madura JA, Fogel E, Swack ML, Kopecky KK. Classification and management of perforations complicating endoscopic sphincterotomy. Surgery. 1999;126:658-663; discussion 664-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Kim BS, Kim IG, Ryu BY, Kim JH, Yoo KS, Baik GH, Kim JB, Jeon JY. Management of endoscopic retrograde cholangiopancreatography-related perforations. J Korean Surg Soc. 2011;81:195-204. [PubMed] |
| 15. | Kwon W, Jang JY, Ryu JK, Kim YT, Yoon YB, Kang MJ, Kim SW. Proposal of an endoscopic retrograde cholangiopancreatography-related perforation management guideline based on perforation type. J Korean Surg Soc. 2012;83:218-226. [PubMed] |
| 16. | Ruiz-Tovar J, Lobo E, Sanjuanbenito A, Martínez-Molina E. Case series: pneumoretroperitoneum secondary to duodenal perforation after endoscopic retrograde cholangiopancreatography. Can J Surg. 2009;52:68-69. [PubMed] |
| 17. | Krishna RP, Singh RK, Behari A, Kumar A, Saxena R, Kapoor VK. Post-endoscopic retrograde cholangiopancreatography perforation managed by surgery or percutaneous drainage. Surg Today. 2011;41:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Lee TH, Bang BW, Jeong JI, Kim HG, Jeong S, Park SM, Lee DH, Park SH, Kim SJ. Primary endoscopic approximation suture under cap-assisted endoscopy of an ERCP-induced duodenal perforation. World J Gastroenterol. 2010;16:2305-2310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Solomon M, Schlachterman A, Morgenstern R. Iatrogenic duodenal perforation treated with endoscopic placement of metallic clips: a case report. Case Rep Med. 2012;2012:609750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kwon CI, Song SH, Hahm KB, Ko KH. Unusual complications related to endoscopic retrograde cholangiopancreatography and its endoscopic treatment. Clin Endosc. 2013;46:251-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Salord S, Gornals JB, Maisterra S, Pons C, Busquets J, Fabregat J. Endoscopic closure of duodenal perforation with an over-the-scope clip during endoscopic ultrasound-guided cholangiopancreatography. Rev Esp Enferm Dig. 2012;104:489-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Dubecz A, Ottmann J, Schweigert M, Stadlhuber RJ, Feith M, Wiessner V, Muschweck H, Stein HJ. Management of ERCP-related small bowel perforations: the pivotal role of physical investigation. Can J Surg. 2012;55:99-104. [PubMed] |
| 23. | Palanivelu C, Jategaonkar PA, Rangarajan M, Anand NV, Senthilnathan P. Laparoscopic management of a retroperitoneal duodenal perforation following ERCP for periampullary cancer. JSLS. 2008;12:399-402. [PubMed] |
| 24. | Enns R, Eloubeidi MA, Mergener K, Jowell PS, Branch MS, Pappas TM, Baillie J. ERCP-related perforations: risk factors and management. Endoscopy. 2002;34:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Mao Z, Zhu Q, Wu W, Wang M, Li J, Lu A, Sun Y, Zheng M. Duodenal perforations after endoscopic retrograde cholangiopancreatography: experience and management. J Laparoendosc Adv Surg Tech A. 2008;18:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Park WY, Cho KB, Kim ES, Park KS. A case of ampullary perforation treated with a temporally covered metal stent. Clin Endosc. 2012;45:177-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Vezakis A, Fragulidis G, Nastos C, Yiallourou A, Polydorou A, Voros D. Closure of a persistent sphincterotomy-related duodenal perforation by placement of a covered self-expandable metallic biliary stent. World J Gastroenterol. 2011;17:4539-4541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Genzlinger JL, McPhee MS, Fisher JK, Jacob KM, Helzberg JH. Significance of retroperitoneal air after endoscopic retrograde cholangiopancreatography with sphincterotomy. Am J Gastroenterol. 1999;94:1267-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |