Published online Mar 26, 2025. doi: 10.12998/wjcc.v13.i9.96897
Revised: October 8, 2024
Accepted: December 2, 2024
Published online: March 26, 2025
Processing time: 208 Days and 20.6 Hours
Alagille syndrome is a multisystem disease that results in various vascular ano
A 34-year-old woman with a history of Alagille syndrome who underwent successful atrial septal defect with partial anomalous pulmonary veins and patent ductus arteriosus repair, as well as left pulmonary artery catheterization and stenting in childhood due to pulmonary stenosis. The patient was without any respiratory symptoms and was a dancer prior to contracting COVID-19. Several weeks after her COVID-19 infection, she developed left pulmonary artery stent thrombosis and subsequent symptomatic pulmonary hypertension. A treatment strategy of anticoagulation alongside pharmacological agents for pulmonary hypertension for 3 months followed by balloon pulmonary artery angioplasty to reopen the stenosis was unsuccessful.
In the era of COVID-19, patients with pulmonary vascular malformations and endovascular stents are at an increased risk for chronic thromboembolic disease. Patients may benefit from prophylactic antiplatelet or anticoagulation therapy. Stent thrombosis is a devastating phenomenon and should be treated urgently and aggressively with balloon pulmonary angioplasty, and/or a thrombolytic agent.
Core Tip: We suggest that in this era of coronavirus disease 2019, patients with endovascular stents and known pulmonary vessel malformations be considered for preventive anticoagulation or antiplatelet therapy, since a thrombotic event in such patients may result in secondary pulmonary hypertension and devastating, irreversible damage. Furthermore, patients with thrombosed stents should be treated aggressively and quickly once a clot is diagnosed.
- Citation: Izhakian S, Korlansky M, Rosengarten D, Bruckheimer E, Kramer MR. Pulmonary artery stent thrombosis and symptomatic pulmonary hypertension following COVID-19 infection in Alagille patient: A case report. World J Clin Cases 2025; 13(9): 96897
- URL: https://www.wjgnet.com/2307-8960/full/v13/i9/96897.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i9.96897
Alagille syndrome is an autosomal dominant disease characterized by cardiac, pulmonary, skeletal, liver, kidney, and eye anomalies, as well as a characteristic facial dysmorphism. It is thought to result from a defective Notch signaling pathway. Mutations in JAG1 are implicated in approximately 90% of cases, with mutations in NOTCH2 and JAG1 de
In February of 2022, a 34-year-old woman was evaluated in our hospital for shortness of breath of 5 days’ onset.
The patient experienced a cough in January 2022 and was diagnosed with coronavirus disease 2019 (COVID-19) infection. Her disease course was mild with no dyspnea and required no medical treatment or oxygen supplementation. Her symptoms improved and she returned to her daily routine for several weeks. One month after her COVID-19 diagnosis she presented to our emergency department with acute dyspnea and chest pain. On evaluation she had an oxygen sa
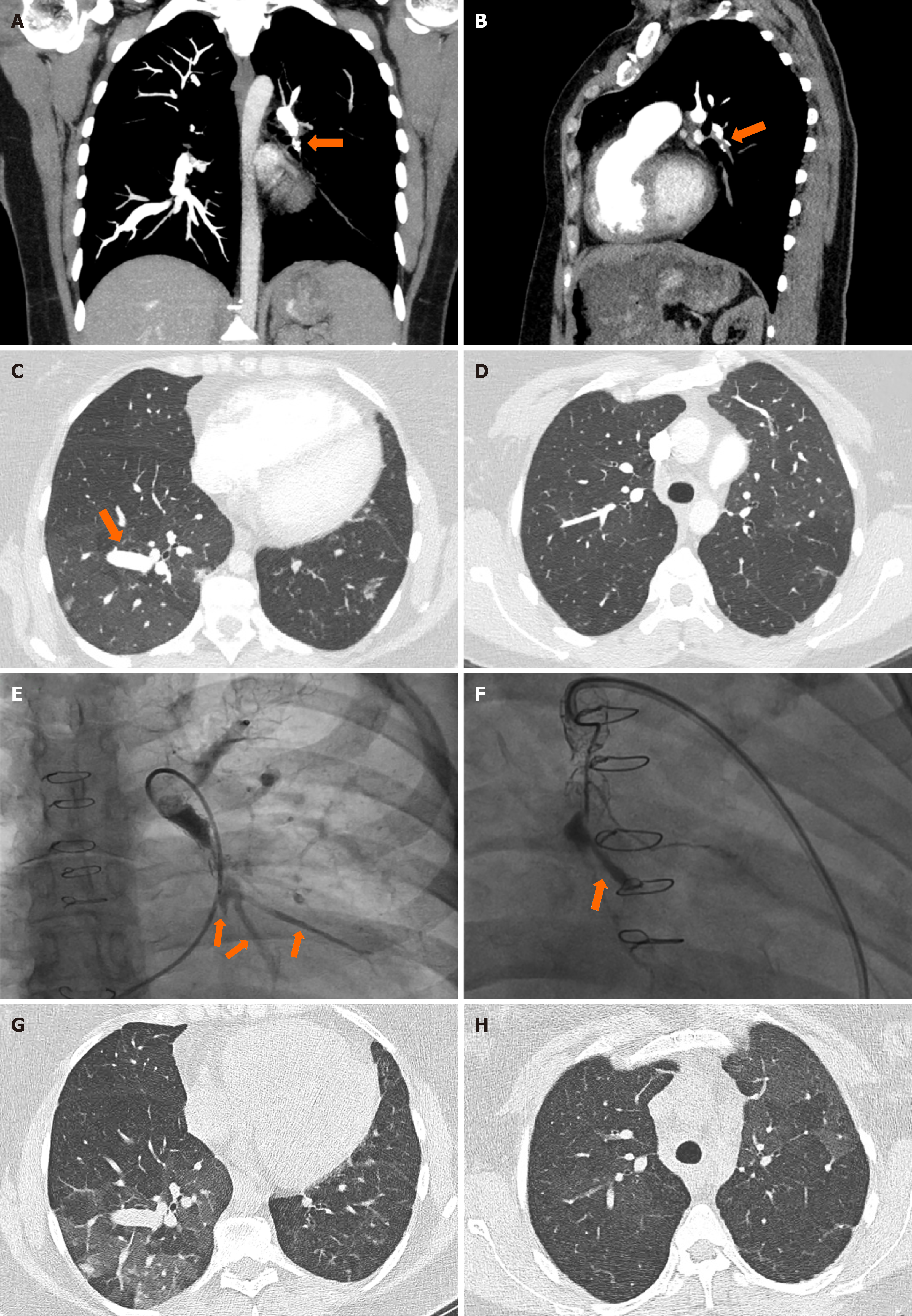
Four months later she continued to suffer from dyspnea on exertion and was still requiring supplemental oxygen for exertion and during sleep. A 6-minute walk test demonstrated a desaturation from 97% at the start to 79% after 445 meters on room air. An echocardiogram demonstrated an ejection fraction of 65%, no leak around the ASD site, systolic pulmonary pressure of 34 mmHg, and normal size and respiratory collapse of the inferior vena cava. There was flattening of the interventricular septum, suggesting right ventricular overload. A right heart catheterization (RHC) was done in October 2022, which confirmed significant precapillary pulmonary hypertension (PHTN) with a mean pulmonary artery pressure (MPAP) of 62 mmHg (normal range < 20 mmHg), a normal pulmonary capillary wedge pressure of 14 mmHg (normal range < 15 mmHg), and pulmonary vascular resistance (PVR) of 20 Wood units (normal range < 2 Wood units). Pulmonary artery angiography showed a thrombosed and fractured stent in the LPA. No collateral vasculature was observed, suggesting a recent occlusion. Balloon dilation to just 2.5 mm was performed due to concerns of pulmonary congestion. After contrast material was injected, three intact vessels were visualized distal to the stent (Figure 1E).
This patient’s childhood history includes an ASD with partial anomalous pulmonary veins (PAPVR), PDA, and LPA stenosis. She underwent surgical repair of her ASD, PAPVR and PDA at 2 years old. At age 9 she underwent balloon dilation and stent placement in the LPA, followed by re-stenting at age 15. During her RHC at age 15, her MPAP was 30 mmHg. She is also known to have chronically elevated GGT of 73 IU/L (normal range 0-30 IU/L) at baseline, and chronic kidney disease with a baseline creatinine of 1.45 mg/dL (normal range 0.6-1.1 mg/dL). Alagille syndrome diagnosis was confirmed after a JAG1 mutation was noted on genetic testing in 2023. Prior to her COVID-19 infection, she led an active life, was a dancer and worked full time as a kindergarten teacher.
No Alagille syndrome in the family, parents and siblings are healthy.
The patient presented to the emergency department in February 2022. Physical examination showed an oxygen saturation of 93% on room air, 18 breaths per minute. Lungs were clear bilaterally on auscultation with no crackles or wheezing. No central pallor or cyanosis were noted. The abdomen was soft and non-tender to palpation. No erythema or edema concerning for deep vein thrombosis (DVT) were noted in the lower extremities.
During her last outpatient follow up in March 2023, her physical examination was not significantly changed. Oxygen saturation was 94% on room air at rest, respirations were 16 per minute. Lungs were clear bilaterally on auscultation. Abdomen was soft and non-tender to palpation. No signs of DVT. She uses 2 L of oxygen via nasal cannula while ambulating.
During her initial evaluation at the emergency department in January 2022, the complete blood count was significant for mild leukocytosis with a white blood cell count of 11.6 K/micl (normal range 4.5-11 K/micl), and a neutrophil count of 9.4 K/micl (normal range 1.8-7.7 K/micl). A complete metabolic panel demonstrated no electrolyte abnormalities and was consistent with her baseline labs. Coagulation studies were remarkable for a D-Dimer of 995 ng/mL (normal range < 500 ng/mL) and was cause to perform a CT angiogram.
During outpatient follow-up, her pulmonary function test (PFT) in April 2022 showed moderate restrictive lung disease (FEV1 = 56%, FVC = 59%, FEV1/FVC = 0.82%, TLC = 72%) with a moderately lower diffusion capacity (DLCO = 56%). There has been no significant change in her PFTs throughout her follow-up with the outpatient lung clinic over the past 16 months. There are no baseline PFTs to compare to from before her COVID-19 infection.
During the initial evaluation in February 2022, a CT angiogram showed a thrombus at the site of a stent in the left lower lobe pulmonary artery (Figure 1A and B) with impaired flow to the distal arteries and mosaicism of lung parenchyma (Figure 1C).
Three blood vessels were visualized distal to the stent during her first RHC in October 2022 (Figure 1E), but none were visualized after contrast injection during her second RHC in February 2023 (Figure 1F).
The final diagnosis was pulmonary artery stent thrombosis with consequent worsening PHTN following COVID-19 infection in a patient with Alagille syndrome.
Following the RHC in October of 2022, a multidisciplinary discussion was held with PHTN specialists, interventional cardiologists, and cardiothoracic surgeons. A treatment strategy was formulated that included lowering her PHTN using pharmacological agents for 3 months, followed by another RHC to attempt reopening the occluded stent, all while maintaining anticoagulation using apixaban. She was started on sildenafil 20 mg PO tid and macitentan 10 mg PO daily. Approximately one month into treatment, the patient reported worsening dyspnea both at rest and during exertion. A 6-minute walk test demonstrated worsening hypoxemia and distance, with desaturations from 91% at the start to 72% on room air at the end of 240 meters. Sildenafil and macitentan were discontinued.
During a follow-up multidisciplinary meeting, the possibility of post-COVID pneumonitis was considered as a con
A follow-up RHC 3 months later showed a complete occlusion of the endovascular stent with no distal arteries visualized after contrast injection (Figure 1F). All attempts to recanalize the LPA with microcatheters failed. However, there was an improvement of the MPAP to 45 mmHg, PVR to 14 mmHg, and a stable PCWP of 15 mmHg. Following the heart catheterization, she was started on riociguat 5 mg PO bid but experienced urticaria and rash that did not improve on anti
Alagille syndrome is a multisystem disease with potentially devastating manifestations, particularly of the cardio
Based on our experience treating this patient, we suggest that COVID-19 infection places patients with endovascular stents and known pulmonary vessel malformations at high risk for stent thrombosis and secondary pulmonary hy
| 1. | Mitchell E, Gilbert M, Loomes KM. Alagille Syndrome. Clin Liver Dis. 2018;22:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Yuan SM. Pulmonary artery pathologies in Alagille syndrome: a meta-analysis. Postepy Kardiol Interwencyjnej. 2022;18:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Kohut TJ, Gilbert MA, Loomes KM. Alagille Syndrome: A Focused Review on Clinical Features, Genetics, and Treatment. Semin Liver Dis. 2021;41:525-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 4. | Kichloo A, Dettloff K, Aljadah M, Albosta M, Jamal S, Singh J, Wani F, Kumar A, Vallabhaneni S, Khan MZ. COVID-19 and Hypercoagulability: A Review. Clin Appl Thromb Hemost. 2020;26:1076029620962853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 5. | Palmerini T, Biondi-Zoccai G, Della Riva D, Stettler C, Sangiorgi D, D'Ascenzo F, Kimura T, Briguori C, Sabatè M, Kim HS, De Waha A, Kedhi E, Smits PC, Kaiser C, Sardella G, Marullo A, Kirtane AJ, Leon MB, Stone GW. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 739] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 6. | Simeone B, Maggio E, Schirone L, Rocco E, Sarto G, Spadafora L, Bernardi M, D'Ambrosio L, Forte M, Vecchio D, Valenti V, Sciarretta S, Vizza CD. Chronic Thromboembolic Pulmonary Hypertension: the therapeutic assessment. Front Cardiovasc Med. 2024;11:1439411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Kuo WT, Sista AK, Faintuch S, Dariushnia SR, Baerlocher MO, Lookstein RA, Haskal ZJ, Nikolic B, Gemmete JJ. Society of Interventional Radiology Position Statement on Catheter-Directed Therapy for Acute Pulmonary Embolism. J Vasc Interv Radiol. 2018;29:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |