Published online Mar 16, 2025. doi: 10.12998/wjcc.v13.i8.97677
Revised: October 9, 2024
Accepted: November 25, 2024
Published online: March 16, 2025
Processing time: 182 Days and 3.3 Hours
Since the advent of the 20th century, alongside the progression of medical science and technological advancements, immunotherapy has emerged as a pivotal thera
Arimab (camrelizumab), a monoclonal antibody targeting programmed death protein 1 (PD-1), disrupts the PD-1/ programmed death ligand 1 (PD-L1) inter
The increasing adoption of immune checkpoint inhibitors in clinical practice has prompted substantial concerns about their safety profile. A wide range of immune-related adverse events and corresponding management stra
Core Tip: The use of immune checkpoint inhibitors has complemented and enriched the treatment options other than radiation and chemotherapy, and has been recognized by the tumor population. The growing availability and clinical use of immune checkpoint inhibitors have raised significant clinical concerns regarding their safety. Timely identification and diagnosis, coupled with multidisciplinary consultation and the prompt administration of immunosuppressants, can effectively address severe immune-related adverse reactions. This report outlines the treatment course and outcome of a case featuring immune-related cutaneous adverse reactions, hope to provide different perspectives for immunotherapy and care of tumors to get the attention of peers and promote the development of related technologies.
- Citation: Jiang YJ, Wu L, Yang X, Pu Y, Ning BJ, Peng N, Zhu XJ. Dermatitis bullosa caused by the immune checkpoint inhibitor camrelizumab: A case report. World J Clin Cases 2025; 13(8): 97677
- URL: https://www.wjgnet.com/2307-8960/full/v13/i8/97677.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i8.97677
Camrelizumab, a programmed cell death protein 1 (PD-1) inhibitor, is a novel humanised high-affinity IgG4-kappa monoclonal antibody, recently approved in China for patients with locally advanced or metastatic oesophageal squamous carcinoma unresponsive to first-line chemotherapy. This approval stems from the results of the Class I ESCORT study[1]. Despite its market presence since 2019, comprehensive data on camrelizumab-associated adverse reactions remain scarce. Official reports indicate a 2.8% occurrence of immune-related cutaneous adverse events, with 0.7% classified as grade 3. This study discusses a case involving grade 3 dermatitis bullosa following camrelizumab administration.
Rash appeared on both hands five days after receiving immunotherapy for esophageal cancer, approximately one month prior.
Demographic details and medical history of the patient were as follows: The patient, a 67-year-old male with a height of 160 cm, weight of 47 kg, and a BSA of 1.46 m², presented with a 20-year smoking history (12-14 cigarettes/day). His Eastern Cooperative Oncology Group score was 2, nutrition score 0, and pain score 3. He reported difficulty swallowing dry or hard foods, with no accompanying symptoms such as hoarseness or coughing when consuming liquids. There was no genetic predisposition noted, and the patient demonstrated strong family support and active engagement in treatment. A gastroscopic biopsy confirmed oesophageal squamous cell carcinoma. In early 2022, he received two cycles of camrelizumab combined with paclitaxel and carboplatin, alongside 30 sessions of oesophageal radiotherapy. Post-radiotherapy, chest-enhanced magnetic resonance imaging (MRI) indicated disease progression, with no surgical options recommended. The patient was subsequently diagnosed with stage IVb oesophageal squamous carcinoma (cT4bNxM1). Following this, he underwent two additional cycles of camrelizumab monotherapy on 8 and 29 June 2022 (Table 1). His dysphagia showed improvement, and the disease was classified as stable. However, 15 days after the fourth cycle of camrelizumab, the patient developed a pruritic erythematous rash on his hands, feet, and scrotum, leading to a prelimi
| Drug name | Usage, dosage, and route of administration | Therapy duration |
| Camrelizumab | 200 mg ivgtt qd | June 8, 2022 |
| 200 mg ivgtt qd | June 29, 2022 | |
| Hydrotalcite chewable tablets | 0.5 g po tid | July 22, 2022 - August 1, 2022 |
| Methylprednisolone sodium Succinate injection | 80 mg iv qd | July 21, 2022 - July 27, 2022 |
| 80 mg iv mos | July 22, 2022 | |
| 80 mg iv mos | July 23, 2022 | |
| 60 mg iv mos | July 24, 2022 | |
| 60 mg iv mos | July 25, 2022 | |
| 40 mg iv mos | July 26, 2022 | |
| 20 mg iv mos | July 27, 2022 | |
| Dexamethasone sodium phosphate injection | 18 mg ivgtt mos | July 28, 2022 |
| 15 mg ivgtt mos | July 29, 2022 | |
| 15 mg ivgtt mos | July 30, 2022 | |
| 11.25 mg ivgtt mos | July 31, 2022 | |
| 11.25 mg ivgtt mos | August 1, 2022 | |
| Methylprednisolone | po qd | Discharge medication |
The patient had maintained generally good health, with a past diagnosis of hepatitis B confirmed at our hospital in January 2022, for which entecavir had been regularly administered. No history of tuberculosis, malaria, or other infec
Personal history: The patient was born and had resided in the same local area for an extended period, with no history of residence in epidemic zones, exposure to contaminated water sources, radiation, toxins, or harmful substances. The patient smoked 12-14 cigarettes daily for 40 years before quitting following illness and consumed 2-3 Liang of alcohol daily for the same duration, ceasing after the onset of illness. There was no involvement in metallurgy or tourism. Regarding COVID-19, the patient had no history of living in or visiting epidemic zones, nor contact with individuals returning from such areas. There had been no symptoms of fever, fatigue, dry cough, diarrhoea, sore throat, or respi
Family history: The father’s cause of death remained unknown, while the mother died from bone metastasis of a malig
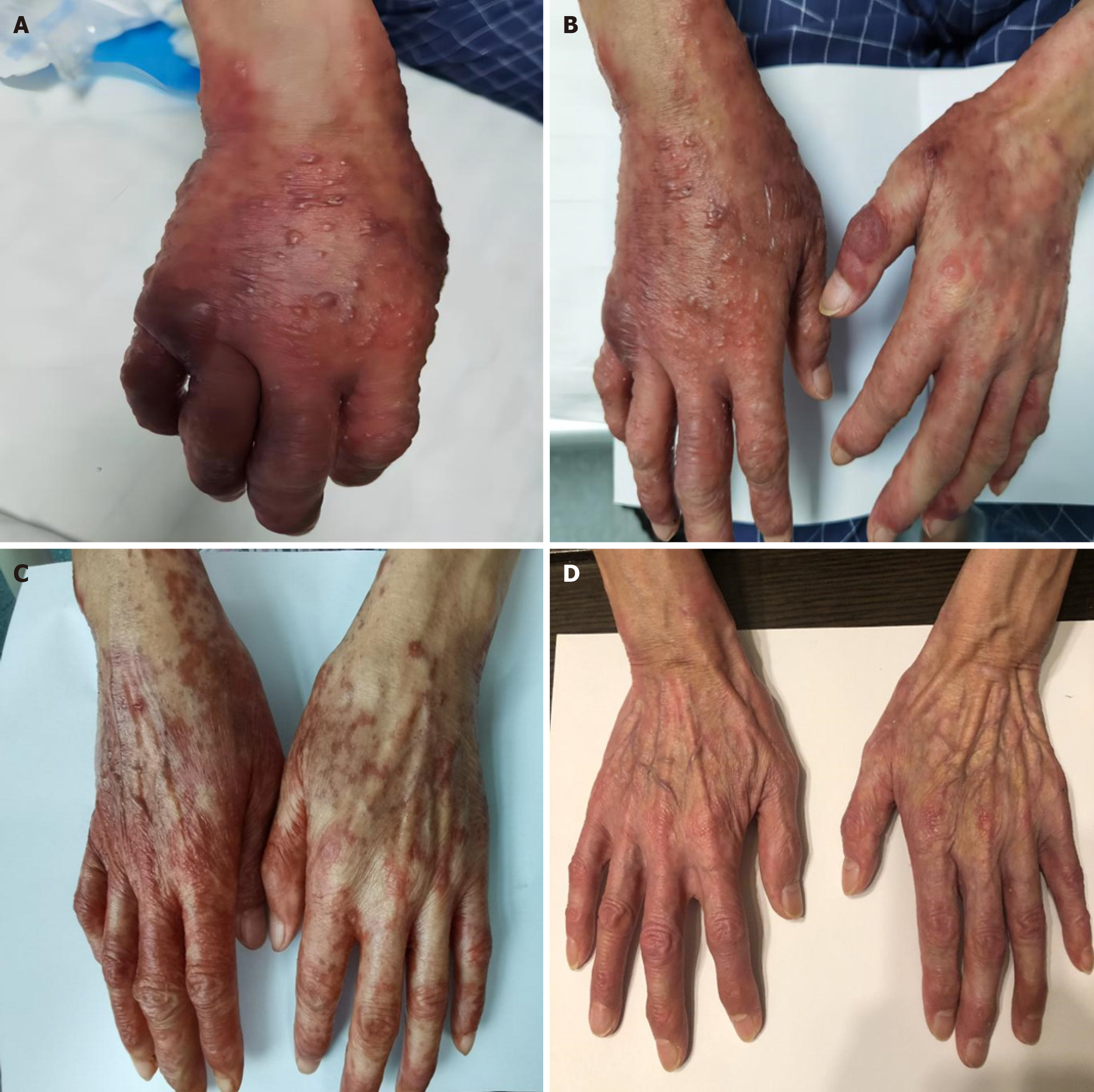
The physical examination revealed scattered erythematous patches on the palms and dorsum of the hands, which were painful upon compression. Blisters of varying sizes, from 2 cm × 3 cm to less than 0.5 cm × 0.5 cm, were dispersed on the dorsum of both hands. Linear erythema and blisters measuring 3 cm × 3 cm and 1.5 cm × 2 cm, along with multiple smaller blisters (< 0.5 cm × 0.5 cm), were present on the right upper limb and shoulder-neck area. Small erythematous lesions with swelling were noted on both feet and the scrotum. The extent of blister involvement covered over 30% of the BSA. No palpable superficial lymphadenopathy was identified. Lung auscultation revealed clear breath sounds, with no evidence of dry or moist rales. Heart auscultation showed a regular rhythm without murmurs across the valve areas. The abdomen was soft, non-tender, and exhibited no rebound pain or palpable masses. Liver, spleen, and subcostal palpation, along with abdominal percussion, were unremarkable, and no signs of mobile dullness were observed. Bowel sounds were normal, and no oedema was present in the lower extremities.
The laboratory results revealed a squamous epithelial cell carcinoma antigen level of 5.07 ng/mL. Blood tests, including markers for C-reactive protein, liver and kidney function, electrolytes, coagulation parameters (six indicators), cardiac damage markers, thyroid function, as well as the analysis of the drainage fluid culture and drug susceptibility testing, all fell within normal limits.
Chest MRI with contrast revealed lower esophageal squamous cell carcinoma (cT4bNxM1, stage IVb).
After consultations with dermatologists and pharmacists, grade 3 dermatotoxicity was confirmed, determined by physical examination, clinical presentation, and classification according to the Common Terminology Criteria for Adverse Events (CTCAE) 4.0 criteria.
Lower esophageal squamous cell carcinoma (cT4bNxM1, stage IVb), and immune checkpoint inhibitor-induced rash.
Consisted of intravenous methylprednisolone sodium succinate combined with topical drug applications. Concurrent interventions included gastric mucosal protection and energy supplementation. After one week, a marked reduction in dermatitis was observed on the hands and feet, with local blisters decreasing in size or partially resolving. By day 10, further improvement in dermatitis was noted. Following 11 days of treatment, routine blood tests indicated normal values for hematologic parameters, liver and kidney function, and cardiac injury markers. Upon discharge, the patient continued oral methylprednisolone sodium succinate, with complete symptom resolution 15 days post-discharge (Table 1, Figure 1A-D).
Symptoms subsided 15 days post-discharge, with complete skin recovery observed at the one-month follow-up.
ESCORT-1st marks the first investigation into first-line therapy for advanced oesophageal squamous cell carcinoma, combining immunotherapy with chemotherapy. The study shows that camrelizumab, when combined with paclitaxel and cisplatin, offers significant survival benefits for previously untreated patients compared to placebo plus chemo
Cutaneous adverse reactions are a common category of irAEs, with varying levels of skin toxicity observed among different immune checkpoint inhibitors. The incidence of PD-1 inhibitor-associated irAEs is reported between 13% and 20%, with the majority being mild, while less than 2.5% are classified as grades 3-4[8]. In this study, camrelizumab-related immune-mediated cutaneous reactions occurred in approximately 2.8% of cases, with grade 3 events accounting for 0.7%. The median onset time was 1.6 months (range: 0–14.4 months), and the median duration was 2.0 months (range: 0-16 months). Notably, 64% of patients experienced disease remission, with a median time to remission of 2.6 months (range: 0–16.5 months). The incidence of dermatitis related to camrelizumab was 16% (9/93 patients), with all cases classified as grades 1-2. Following combination chemotherapy (gemcitabine + cisplatin), grade 1 dermatitis was observed in 65% of patients (10/23), while grade 3 dermatitis occurred in 4% (1/23)[9]. The Chinese Society of Clinical Oncology guidelines recommend glucocorticoids for managing grade 3 or higher dermatitis, with tapering after improvement to grade 1, in combination with hormone therapy (Table 2).
| Grading | Description | Recommendation for grade I | Recommendation for grade II | Recommendation for grade III |
| Grade 1 | Asymptomatic; blisters covering < 10% of the body surface area (BSA); topical potent glucocorticoids | Emergency dermatology consultation; routine blood, liver, and kidney function, electrolytes, and C-reactive protein (CRP) tests | ||
| Grade 2 | Painful blisters covering 10%-30% of the BSA; limited daily use of tools | Suspension of immune checkpoint inhibitor (ICI) treatment until the toxicity is < grade I; prednisone/methylprednisolone (0.5–1 mg/kg/d]); routine blood, liver, and kidney function, electrolyte, and CRP tests | Emergency dermatology consultation | |
| Grade 3 | Blisters covering > 30% of the BSA; significant limitation in self-care and daily life; Stevens–Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) | Permanent discontinuation of treatment with ICIs; prednisone/methylprednisolone (1–2 mg/kg/d); hospital admission to the burn ward, intensive care unit monitoring or emergency consultation with a dermatologist, ophthalmologist, and urologist; test for routine blood indicators, liver and kidney functions, electrolyte levels, CRP, complement, and other relevant inflammatory factors | Skin biopsy when necessary | |
| Grade 4 | Blisters covering > 30% of the BSA; concurrent fluid and electrolyte abnormities; lethal SJS or TEN | |||
Multiple adverse events have been observed in clinical trials of paclitaxel albumin, including alopecia, generalized rash, nail changes, hyperpigmentation, and pruritus. Randomized controlled trials in Europe, the United States, and China showed that rash occurred in 8% of European and American patients, compared to 26% of Chinese patients, while pruritus affected 6% and 21% of these populations, respectively. Skin reactions typically appear 2-3 days after initial dosing cycles, resolving either spontaneously or with symptomatic intervention. Carboplatin, by contrast, reports no common cutaneous adverse effects and a low incidence of peripheral neuropathy, such as sensory abnormalities or decreased deep tendon reflexes. Nonetheless, patients with pre-existing sensory impairments, particularly those induced by cisplatin, may experience persistent or worsening symptoms during carboplatin therapy. In this case, the patient developed a red rash accompanied by pruritus on the hands, feet, and scrotum 15 days after receiving camrelizumab. While paclitaxel albumin–related rashes typically manifest within 2-3 days of multiple dosing cycles, this patient had last received paclitaxel albumin approximately 5-6 months prior. At that time, no documented evidence of carboplatin-associated cutaneous adverse reactions was available. Given the delayed timeline and absence of paclitaxel/carboplatin-related bullous rash reports, the introduction of camrelizumab is a more likely cause of the rash. This assessment aligns with the CTCAE 4.0 grading system, under which the rash was classified as grade 3. For patients presenting with grade 3 dermatological toxicity, clinical guidelines recommend discontinuing immune checkpoint inhibitors and conducting a skin biopsy in consultation with a dermatologist. After undergoing multidrug therapy and skin biopsy, this patient was treated with glucocorticoids, antihistamines, and other symptomatic measures. The patient subsequently reported marked symptom improvement, suggesting the therapeutic approach was effective.
The increasing adoption of immune checkpoint inhibitors in clinical practice has prompted substantial concerns about their safety profile. A wide range of immune-related adverse events and corresponding management strategies have been well-documented. Early recognition and accurate diagnosis, combined with interdisciplinary collaboration and swift initiation of immunosuppressive therapy, are essential in managing severe immune-related adverse reactions effectively. This report details the treatment trajectory and outcome of a case involving immune-related cutaneous adverse reactions, providing pertinent clinical insights for future cases.
| 1. | Chen P, Fu C, Shen L, Fei Z, Luo M, Chen Y, Li H. Cost-effectiveness analysis of tislelizumab vs. camrelizumab for the treatment of second-line locally advanced or metastatic esophageal squamous cell carcinoma. BMC Health Serv Res. 2024;24:676. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | LaFleur MW, Muroyama Y, Drake CG, Sharpe AH. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol. 2018;200:375-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 3. | Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 948] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 4. | Medina PJ, Adams VR. PD-1 Pathway Inhibitors: Immuno-Oncology Agents for Restoring Antitumor Immune Responses. Pharmacotherapy. 2016;36:317-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Lee HT, Lee JY, Lim H, Lee SH, Moon YJ, Pyo HJ, Ryu SE, Shin W, Heo YS. Molecular mechanism of PD-1/PD-L1 blockade via anti-PD-L1 antibodies atezolizumab and durvalumab. Sci Rep. 2017;7:5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 6. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 452] [Article Influence: 113.0] [Reference Citation Analysis (1)] |
| 7. | Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, Lenihan D, Onofrei C, Shannon V, Sharma R, Silk AW, Skondra D, Suarez-Almazor ME, Wang Y, Wiley K, Kaufman HL, Ernstoff MS; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1337] [Cited by in RCA: 1412] [Article Influence: 176.5] [Reference Citation Analysis (0)] |
| 8. | Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K; ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv264-iv266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 325] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 9. | Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, Zhang Y, Chen L, Zhou N, Zhao Y, Hou X, Yang Q, Zhang L. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 351] [Article Influence: 50.1] [Reference Citation Analysis (0)] |