Published online Feb 26, 2025. doi: 10.12998/wjcc.v13.i6.98084
Revised: October 12, 2024
Accepted: November 7, 2024
Published online: February 26, 2025
Processing time: 159 Days and 20.1 Hours
This manuscript describes the first known cases of sick sinus syndrome (SSS) associated with the use of anlotinib in non-small cell lung cancer patients, highlighting the need for increased vigilance and cardiac monitoring.
Two patients with non-small cell lung cancer developed SSS after 15 months and 5 months of anlotinib treatment, respectively, presenting with syncope and palpitations. Electrocardiogram confirmed SSS, and different treatment approaches were taken for each patient. One patient received a dual-chamber permanent pacemaker, while the other discontinued the medication and experienced symptom resolution.
Anlotinib can induce SSS, suggesting that cardiac monitoring is crucial during anlotinib treatment. Individualized management strategies are necessary for affected individuals.
Core Tip: This case report presents the first known instances of sick sinus syndrome associated with the use of anlotinib in patients with non-small cell lung cancer, emphasizing the importance of cardiac monitoring during treatment. The report compares two different management approaches for anlotinib-induced sick sinus syndrome, one with a dual-chamber permanent pacemaker and the other with medication discontinuation, providing valuable insights for clinical practice.
- Citation: Fu CF, Yang LF, Tian L, Deng S, Zhang Q, Yao B. Anlotinib-induced sick sinus syndrome: Two case reports. World J Clin Cases 2025; 13(6): 98084
- URL: https://www.wjgnet.com/2307-8960/full/v13/i6/98084.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i6.98084
Anlotinib is a small-molecule, multi-target tyrosine kinase inhibitor that is known to inhibit several receptors, including vascular endothelial growth factor receptor (VEGFR, including VEGFR-1, VEGFR-2, and VEGFR-3), platelet-derived growth factor receptor (PDGFR, including PDGFR-α and PDGFR-β), fibroblast growth factor receptor (FGFR, including FGFR-1, FGFR-2, FGFR-3, and FGFR-4), and c-kit[1,2]. Anlotinib has demonstrated significant antitumor effects through its ability to inhibit tumor angiogenesis and growth. Its low half-maximal inhibitory concentration for these targets confers a better safety profile. Anlotinib is recommended as a third-line treatment for non-small cell lung cancer according to the Chinese Society of Clinical Oncology guidelines. Common adverse reactions to anlotinib treatment include dermatitis, gastrointestinal reactions, hypertension, bleeding, and arrhythmia. Sick sinus syndrome (SSS) is a severe arrhythmia caused by abnormalities in the generation or conduction of impulses from the sinoatrial node, leading to various heart rhythm disturbances. To date, no reports have linked anlotinib to the development of SSS. Herein, we report two cases describing such an association.
Case 1: A 75-year-old female presented with a 1-week history of palpitations and discomfort.
Case 2: An 80-year-old male presented with a 15-day history of palpitations and syncope.
Case 1: The patient had been on targeted therapy with anlotinib (12 mg once daily, orally for 14 days, followed by a 7-day break, in a 21-day cycle).
Case 2: The patient had been on the same regimen of anlotinib as case 1.
Case 1: In 2012, the patient had been diagnosed with lung adenocarcinoma, staged as T1N0M0 stage I (negative for driver genes). She had undergone radical resection of the right middle lobe of the lung. In May 2016, a follow-up examination had revealed a nodule in the right upper lobe, suggesting tumor recurrence, for which she had received first-line and second-line chemotherapy. In March 2019, imaging indicated progressive disease, and she had been prescribed oral anlotinib.
Case 2: In January 2019, the patient had been diagnosed with adenocarcinoma in the upper lobe of the left lung, staged as T1N0M1a stage IVa (negative for driver genes). After first-line and second-line treatments, the condition was indicated as stable disease. In June 2021, he had been prescribed oral anlotinib as maintenance therapy.
No significant personal or family histories for case 1 and case 2.
Case 1: Heart rate of 36 beats per minute.
Case 2: Physical examination findings were not specified in the recorded information.
No laboratory testing data were specified in the recorded information for case 1 and case 2.
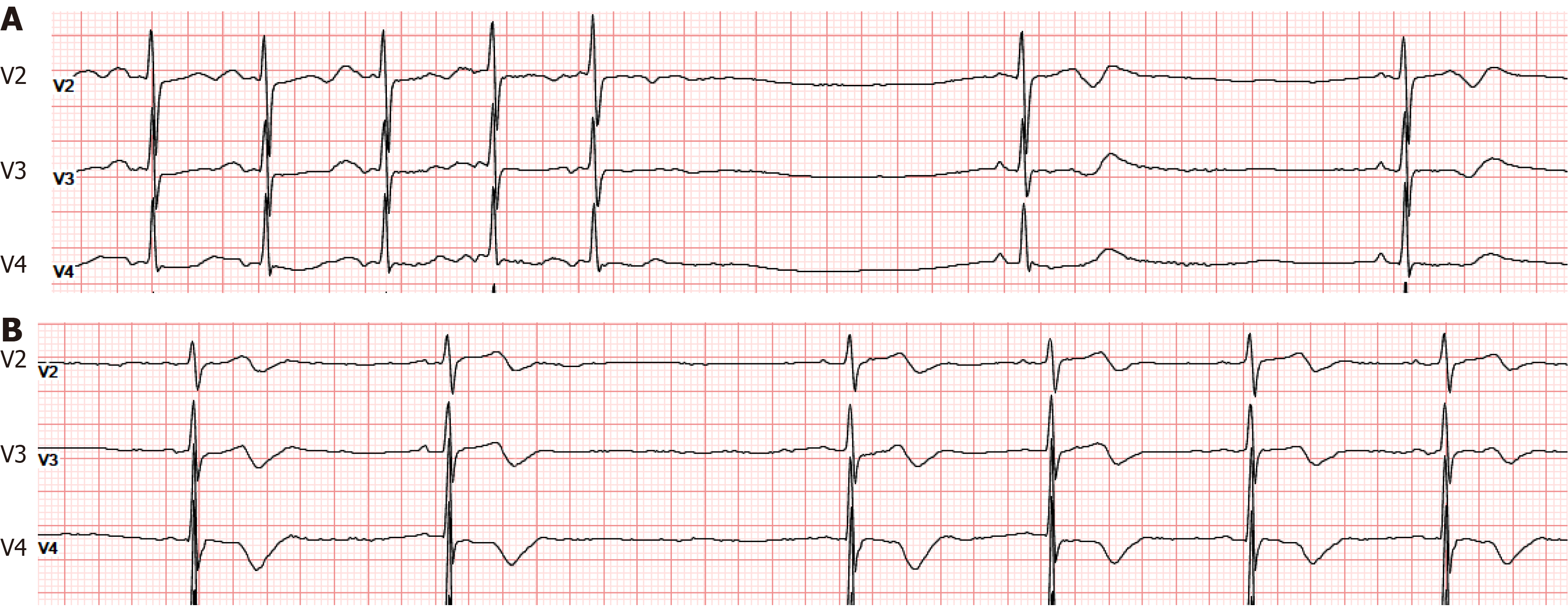
Case 1: Electrocardiogram (ECG) showed sinus bradycardia, T-U fusion, and a prolonged QT interval. A 24-hour Holter monitor revealed a minimum heart rate of 30 beats per minute, with five pauses exceeding 2.0 seconds, the longest RR interval being 2.041 seconds, widespread ST-T changes, and a prolonged QT interval (Figure 1A).
Case 2: ECG showed sinus bradycardia, T-U fusion, and a prolonged QT interval. A 24-hour Holter monitor indicated the slowest heart rate at 27 beats per minute, with 211 pauses greater than 2.0 seconds, the longest RR interval being 2.039 seconds, widespread ST-T changes across the leads, and a prolonged QT interval (Figure 1B).
Both case 1 and case 2 were negative for driver genes.
For both cases, cardiology consultation led to the diagnosis of SSS.
Case 1 and case 2 were both diagnosed with SSS associated with anlotinib use.
The patient underwent implantation of a dual-chamber permanent pacemaker in May 2020.
The patient’s family refused cardiac pacemaker treatment. The medication was discontinued, leading to gradual symptom resolution.
Postoperative ECG follow-ups showed pacemaker rhythms, and the patient remains alive and well.
Symptoms gradually disappeared after discontinuation of the medication, and an ECG performed 2 months later showed a return to a normal rhythm. The patient has been followed up to the present and is alive.
Neither patient had a prior history of hypertension, arrhythmias, or heart failure. After receiving anlotinib for lung adenocarcinoma for 15 months and 5 months, respectively, they presented with syncope accompanied by palpitations. ECG findings suggested SSS, which was suspected to be related to anlotinib. The patients had each previously been treated with pemetrexed disodium, cisplatin, carboplatin, and recombinant human endothelial inhibitors. While the labels for these drugs list various arrhythmias as potential side effects, such as supraventricular arrhythmias from pemetrexed disodium, sinus tachycardia or atrioventricular block from recombinant human endothelial inhibitors, tachycardia from cisplatin, and heart failure or myocardial infarction from carboplatin, neither patient had experienced palpitations or syncope while on these medications, nor were there any ECG signs of sinus bradycardia, T-U fusion, or prolonged QT intervals. After switching to oral anlotinib, SSS occurred after 15 months and 5 months, respectively, suggesting that the previous drugs were not the cause. Anlotinib is known to frequently cause sinus tachycardia and, less commonly, sinus bradycardia and arrhythmias, with occasional cases of atrial fibrillation. Based on the patients’ medication history and the timing of onset, it appears likely that anlotinib induced the SSS.
Anlotinib is a small-molecule, multi-target tyrosine kinase inhibitor that inhibits VEGFR (VEGFR-1, VEGFR-2, and VEGFR-3), PDGFR (PDGFR-α and PDGFR-β), FGFR (FGFR-1, FGFR-2, FGFR-3, and FGFR-4), and c-kit[1,2]. Its mechanism of action, including the inhibition of tumor angiogenesis and growth, offers a significant therapeutic advantage, with low half-maximal inhibitory concentration values leading to better safety. It was approved in China in May 2018 for third-line treatment of non-small cell lung cancer with negative driver genes. Anlotinib has not been linked to SSS in clinical trials[3] nor in real-world studies[4]. The two patients in this report opted for different treatment approaches for SSS, providing valuable insights into the management of anlotinib-induced SSS in lung cancer patients. Case 1 received a dual-chamber permanent pacemaker with no further palpitations noted during follow-up. Case 2 opted against pacemaker therapy and discontinued the use of anlotinib. After stopping the medication, his symptoms resolved and a follow-up ECG demonstrated a return to sinus rhythm.
In summary, discontinuing the drug and close monitoring may be sufficient to treat anlotinib-induced SSS, and cardiac pacemaker therapy is a possible intervention when necessary.
| 1. | Beedie SL, Mahony C, Walker HM, Chau CH, Figg WD, Vargesson N. Shared mechanism of teratogenicity of anti-angiogenic drugs identified in the chicken embryo model. Sci Rep. 2016;6:30038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, Zhao F, Ahmad R, Zhao J. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 429] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 3. | Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu Y, Feng J, Zhang S, Ba Y, Zhao Y, Liu Y, Bai C, Bai Y, Tang Y, Song Y, He J. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial. Cancer Med. 2021;10:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 4. | Zhang K, Ma X, Gao H, Wang H, Qin H, Yang S, Liu X. Efficacy and Safety of Anlotinib in Advanced Non-Small Cell Lung Cancer: A Real-World Study. Cancer Manag Res. 2020;12:3409-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |