Published online Feb 26, 2025. doi: 10.12998/wjcc.v13.i6.97545
Revised: October 30, 2024
Accepted: November 12, 2024
Published online: February 26, 2025
Processing time: 176 Days and 15.5 Hours
Colonoscopy represents a safe procedure that is widely used in medical practice either to diagnose or treat various gastrointestinal diseases. During the last few years, the incidence rate of perforations in colonoscopic procedures has increased, especially in therapeutic colonoscopies. The recent advancements in endoscopic techniques and gastrointestinal tumoral resection procedures such as endoscopic mucosal resection, endoscopic full-thickness resection, and endoscopic submucosal dissection (ESD) could be a risk factor for this increased risk. The incidence rate of mortality of serious colonoscopic perforations is 7.1%. The management plan for these perforations starts with conservative treatment in mild cases, endoscopic closure, and surgical management in severe cases. Recently, endoluminal vacuum therapy was found to be effective in the management of colorectal perforations and this has been reported in multiple case reports. This editorial provides an overview of the current guidelines for the management of iatrogenic colorectal perforations. These insights are from the perspectives of endoscopists and gastroenterologists. We also present a management algorithm based on the guidelines of the European Society of Gastrointestinal Endoscopy, the American Gastroenterological Association, and the World Society of Emergency Surgery. We also discussed in brief the use of endoluminal vacuum therapy in colorectal perforations.
Core Tip: In this editorial, we summarize the different current society guidelines and evidence for the management of iatrogenic colorectal perforation, emphasizing the role of endoscopy in perforation management.
- Citation: Tawheed A, Bahcecioglu IH, Yalniz M, Ozercan M, Oral AC, El-Kassas M. Summary of the current guidelines for managing iatrogenic colorectal perforations and the evolving role of endoluminal vacuum therapy. World J Clin Cases 2025; 13(6): 97545
- URL: https://www.wjgnet.com/2307-8960/full/v13/i6/97545.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i6.97545
In a recent case report published in the World Journal of Clinical Cases, Lin and Pu[1] the authors reported the use of endoluminal vacuum therapy for a colonic perforation resulting from the insertion of an erotic toy. This editorial pro
Colonoscopy is a generally safe procedure that could help diagnose and treat colorectal diseases. The most severe complication that can occur during a colonoscopy is iatrogenic colorectal perforation. Colon perforation is the complete disruption of all four layers: Mucosa, submucosa, muscularis propria, and serosa of the colon wall, leading to the presence of air in the abdominal cavity (pneumoperitoneum)[2]. The rates of colonic perforations are as low as 0.066%[3]. However, due to the daily evolution in the era of therapeutic endoscopy, the incidence of perforations in therapeutic colonoscopies could increase up to 0.21% compared to 0.019% in diagnostic colonoscopies[3]. Perforations could be severe to the extent of developing peritonitis and sepsis, with a mortality rate of 7.1% for these severe complications[4].
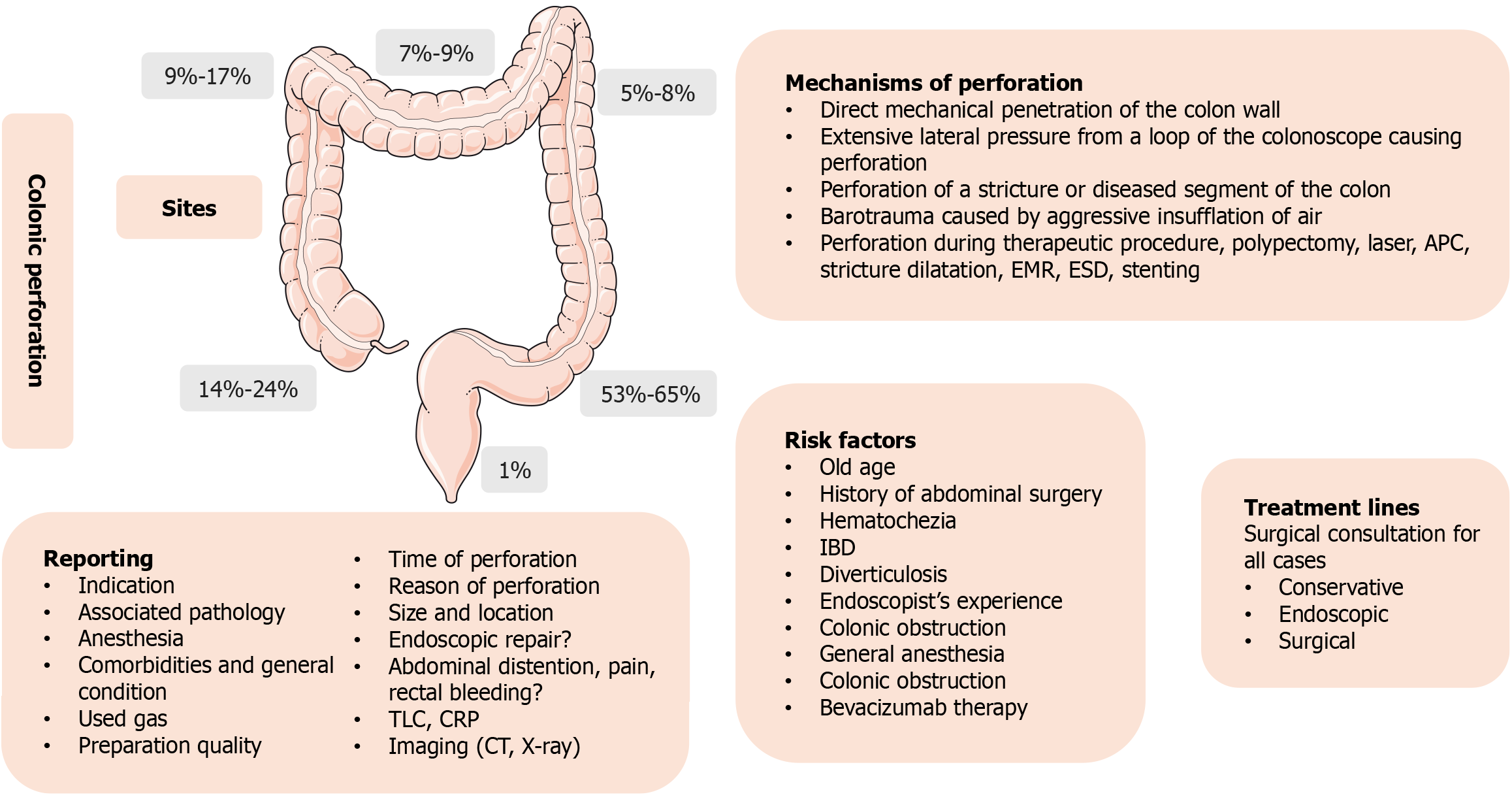
The mechanisms of perforation during colonoscopy include direct mechanical penetration of the colon wall, extensive lateral pressure from a loop of the colonoscope causing perforation, perforation of a stricture or diseased segment of the colon, or barotrauma caused by aggressive insufflation of air[5]. Perforations could also happen during therapeutic procedures that are currently increasing in frequency, such as polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD)[6]. The most common sites for the incidence of perforations are the sigmoid colon (53%-65%), and cecum (14%-24%), followed by ascending colon (9%-17%), transverse colon (7%-9%), then come the descending colon (5%-8%). The least common site is the rectum, with only (1%) (Figure 1)[7].
Multiple reported risk factors increase the risk of colorectal perforation during colonoscopy, including old age, history of abdominal surgery, presence of hematochezia, and those on bevacizumab[8]. Other patients who experience perforations during colonoscopies may have underlying pathology that could lead to this complication, such as inflammatory bowel disease, colonic obstruction, or diverticulosis[7].
In this editorial, we will review the current guidelines for the management of iatrogenic colorectal perforations from the perspective of endoscopists and gastroenterologists. Additionally, we will discuss in brief the use of endoluminal vacuum therapy in colorectal perforations.
In 2020, the European Society of Gastrointestinal Endoscopy (ESGE) updated the original position statement for diagnosing and managing iatrogenic colonic perforations. The group searched the databases for published evidence until October 2019[9].
The statement provided both general and organ-specific recommendations. The authors advocated for a multidisciplinary approach to managing gastrointestinal perforations, emphasizing the importance of a well-defined written protocol for collaboration among surgery, radiology, and gastroenterology departments that includes a list of procedures associated with a high risk of perforation. They also recommended using computed tomography (CT) over plain X-rays for perforation diagnosis, which could prevent delays. The guidelines also stated that all perforations must be reported in detail, including the site and size of the perforation, as well as whether an endoscopic trial to close the perforation has been attempted and whether it was successful.
After colonoscopies, perforations should be suspected in patients present with any of the following symptoms: Abdominal pain, severe distention, difficulty breathing, and signs of surgical emphysema. If left untreated, those perforations could lead to more severe complications, such as septic shock.
If a perforation was not identified during the colonoscopy, it is recommended that the endoscopist maintain a low threshold level of suspicion for any patient displaying symptoms suggestive of perforation, such as abdominal pain, severe distention, difficulty breathing, and signs of surgical emphysema. In the late stages, patients may develop septic shock. Therefore, those patients should be investigated early and undergo a CT scan to confirm the diagnosis, if possible. The guidelines also recommended using a grading system presented by the American Society for Gastrointestinal Endoscopy to classify the severity of the adverse events of endoscopy. There are four grades: (1) Mild: The patient needs hospitalization for 1 to 3 days; (2) Moderate: The patient requires hospitalization for 4 to 9 days; (3) Severe: The patient needs to be hospitalized for more than 10 days, requires surgery, or is admitted to the intensive care unit; and (4) Fatal: The adverse event results in death[10] to multiple factors should be taken into consideration when choosing the management plan for those perforations. These factors include the timing of diagnosis, the amount of leaked bowel contents, the location and size of the defect, the experience of the endoscopist, and the availability of necessary endoscopic tools. When the diagnosis of perforation has been made, it is always recommended to use CO2 instead of room air due to the high absorbability of CO2. This could decrease the need for surgery by minimizing extra insulation in the abdomen. In addition, an attempt to endoscopically close the defect should be made by the endoscopist. Additionally, a trial should be conducted to decompress and relieve the pneumoperitoneum to decrease the possibility of compartmental syndrome. In the event of colonic perforation, immediate measures such as vital sign monitoring, intravenous fluid administration, and the use of broad-spectrum antibiotics are essential for initial management.
Endoscopic management of the perforations requires good preparation of the colon. Small perforations (< 1 cm) could be treated by endoscopic measures using through-the-scope clips (TTSCs). However, over-the-scope clips (OTSCs) are usually more effective in larger defects. Surgical management is usually required for cases with failed conservative or endoscopic management, large perforations, or cases with peritoneal fecal contamination.
The latest recommendations for managing colonic perforations were issued by the American Gastroenterological Association (AGA). The overall recommendations align with those put forth by the ESGE. In this section, we will provide an overview of the AGA recommendations and emphasize any divergent or additional insights[11].
According to the guidelines, endoscopy units should be well-equipped and have a well-trained team to manage such complications with a written protocol on how to manage such cases of perforations. This approach prevents overreactions and panic after the incidence of perforations. Also, it is recommended that the endoscopist notify the team once the perforation is detected to act subsequently. Same as ESGE. AGA recommended shifting the gas to CO2 instead of room air and a trial to suction the fecal matter from the perforated segment area along with the administration of prophylactic antibiotics that are effective against gram-negative bacteria and anaerobic organisms.
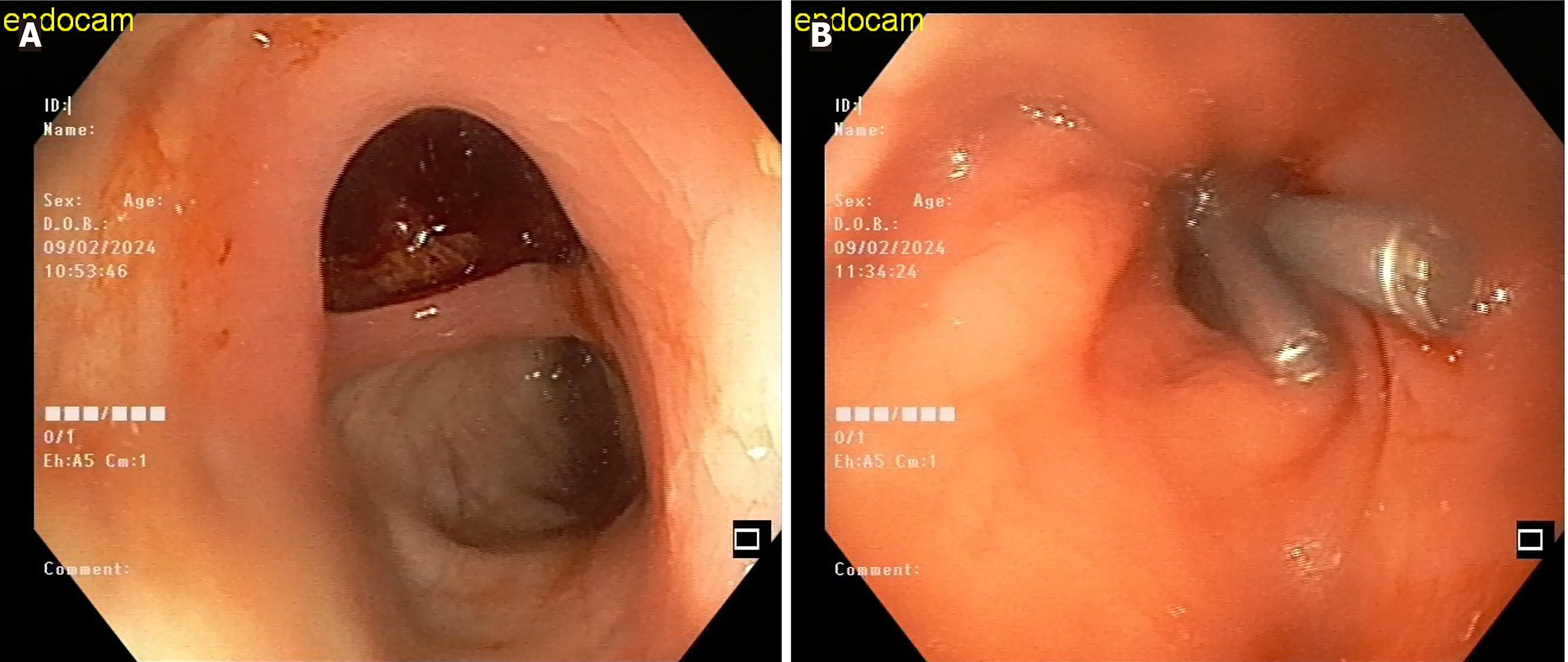
As aforementioned, the most common site for perforations is the sigmoid colon. The typical cause of these perforations is blunt trauma and excessive force by the endoscopist against the colonic wall (Figure 2). They are usually large and rarely missed by the endoscopist. However, the endoscopist should be alert for signs of perforation, such as lumen collapse and the inability to inflate the colon with air. Surgical intervention is typically necessary for this type of perforation in cases of a large tear, patient instability, or delayed diagnosis resulting in peritonitis.
The authors provided recommendations for the removal of polyps using EMR. These included using a viscous solution for submucosal injection, manipulating the polypectomy snare before cutting by partially opening and then reclosing it to avoid cutting the muscularis propria, examining the muscularis propria after cutting to detect any target sign, early detection and endoscopic management of perforations, and utilizing a full thickness resection device in cases of failure of polyp lifting after submucosal injections.
These guidelines introduced endoscopic suturing as a new endoscopic modality for the management of perforations in addition to the existing modalities in ESGE guidelines. Same as previous guidelines, a trial to manage those patients endoscopically only if the patient has a proper bowel preparation and is in stable condition. In cases of perforations in the right colon, the authors suggest utilizing TTSCs due to the inability to use suturing devices with colonoscopies. Additionally, there are risks associated with removing the colonoscopy to install the OTSCs device and reintroducing the colonoscopy. However, in perforations of the rectum, any of the previous endoscopic accessories could be of help.
In case of a complication, this should be discussed with the endoscopy team, the surgical team, and the patient's family should be informed, and the incident should be documented and reported.
The World Society of Emergency Surgery guidelines for managing iatrogenic colonic perforations, which were published nearly 7 years ago, are still considered the most comprehensive and definitive guidelines available. These guidelines addressed 17 critical questions related to iatrogenic colonic perforations. They began with general recommendations to minimize the incidence of these perforations. The guidelines suggest that during colonoscopy training, the trainer endoscopist should have a low threshold for completing the procedure himself or aborting the procedure, particularly in challenging procedures or situations such as obstruction or diverticular disease[7].
Again, another guideline recommended the use of CO2 gas and minimizing insufflation of the colon to prevent barotrauma. Additionally, they advised endoscopists to refrain from pushing the scope excessively against the colon wall. Instead, gentle pushing and a trial to proceed with minimal looping and these measures could be reached through positional adjustments or external support. When performing a polypectomy, the safety margin should be less than 2 cm. Additionally, small polyps measuring less than 5 mm should be removed using cold snare techniques.
The guidelines advise against certain procedures, such as inserting stents for malignant strictures in patients receiving bevacizumab and dilating stenosis in patients with active Crohn's disease or potential fistula presence. Also, for high-risk procedures, it is recommended to have a multidisciplinary backup team comprising endoscopists, radiologists, and surgeons and a well-equipped endoscopy unit. As per the guidelines, the acceptable perforation rates for diagnostic procedures should be below 0.1%, 1% for polypectomies, and less than 7% for patients undergoing colonic stenting. We have outlined the essential data that must be reported and the necessary investigations to be conducted following a perforation in Figure 1.
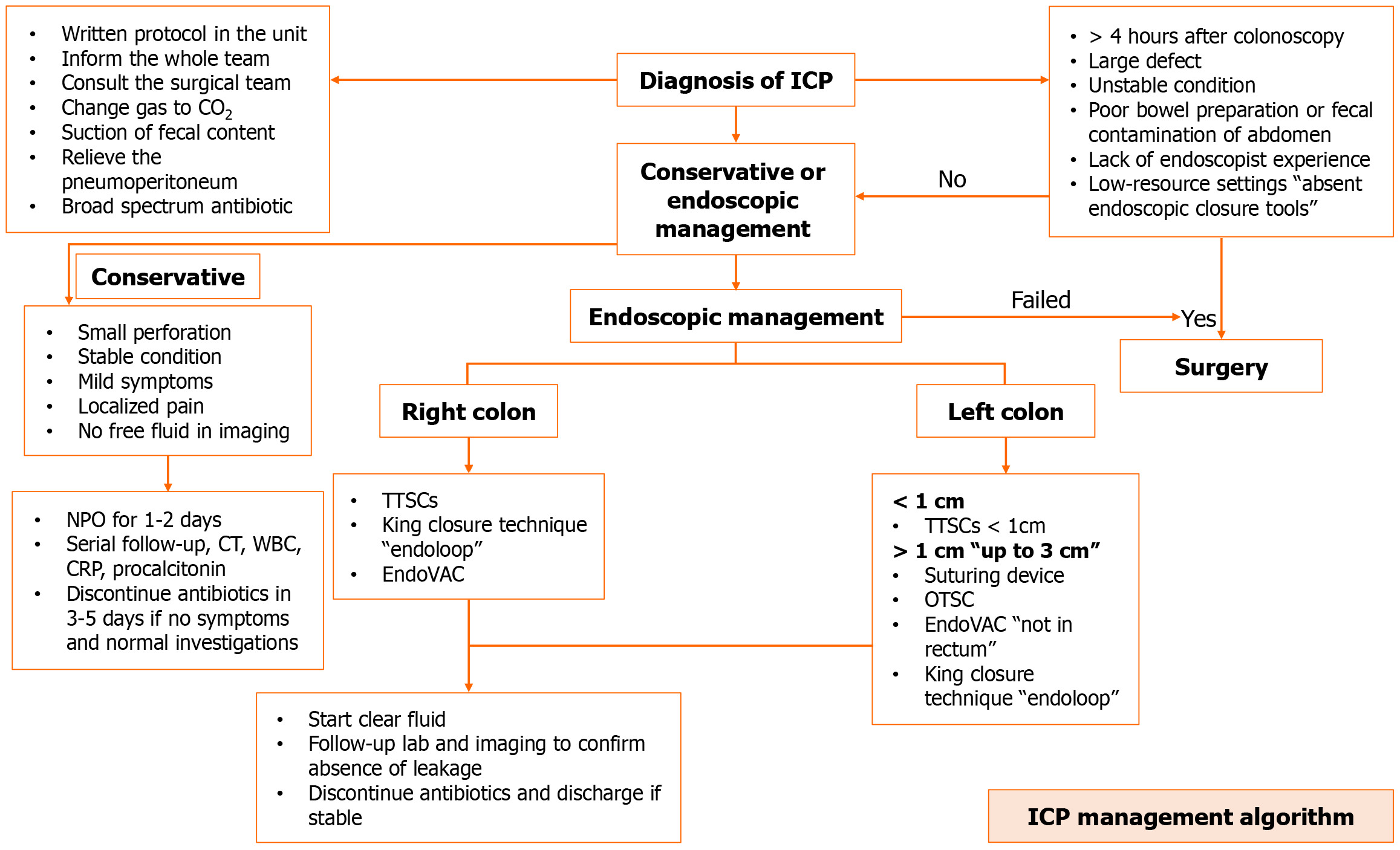
The management of perforations can be approached in the following ways: (1) Conservative management is appropriate for small perforations in stable patients with mild localized symptoms and absence of free fluid in imaging. This could be achieved by bowel rest, clinical follow-up (3-6 hours) including clinical examination and vital signs monitoring, follow-up using serial imaging, intravenous fluid infusion, injection of prophylactic broad-spectrum antibiotics, and a trial to alleviate pneumoperitoneum and this could be helpful during the perforation closure trials and improve patient respiration and also could help in relieving patients’ abdominal pain; (2) Within four hours following the treatment, endoscopic management need to be carried out. The size and nature of the perforation should determine whether endoscopic procedures, such as TTSC, OTSC, or endoscopic suturing devices, are used; and (3) Surgical management is still considered a major part of the management of iatrogenic colorectal perforations. Those who typically need surgical intervention include unstable patients, colons with poor preparation with a significant amount of fecal matter, and those with large perforations that could not be managed endoscopically.
For patients who have undergone successful endoscopic or conservative treatment, it is recommended to conduct follow-up assessments during the hospitalization period. Clinical exams and laboratory monitoring of hemoglobin, white blood cell count, procalcitonin, C-reactive protein, electrolyte levels, and blood urea nitrogen should be part of these evaluations. Furthermore, CT scan imaging is recommended.
While patients who have had effective endoscopic management can start clear fluids right after, those who have had conservative care should remain fasting for one to two days. Patients who have had surgery for repair are free to begin using clear fluids.
The choice of antibiotics should be effective against gram-negative and anaerobic organisms. A short treatment course, especially in the absence of peritonitis or signs of infection (3-5 days), is recommended. In cases where there is an absence of peritonitis or infections in abdominal CT scans, antibiotics may be discontinued to prevent alterations of the gut microbiota.
In severe cases of perforations where surgical repair has been performed, a trial to shorten the antibiotic period should be considered after confirming the resolution of all signs of infection. Additionally, these patients will need to begin thromboprophylaxis during their hospital stay.
Surveillance colonoscopies are recommended following surgical repair of iatrogenic colonic perforation, especially if the initial colonoscopy was incomplete. A repeated colonoscopy should be performed 3-6 months after the full repair of the colonic perforation. The same protocol applies after polypectomy, EMR, or ESD, with a follow-up colonoscopy after 3-6 months to ensure complete removal of the polyp. However, in cases of acute lower gastrointestinal bleeding, colonoscopy is indicated once complete healing of the perforation is achieved.
There is limited evidence on the use of endoscopic band ligation for the treatment of small perforations. This approach may be particularly beneficial in endoscopy units with limited resources that do not have access to OTSCs and where TTSCs (have not been effective in closing the perforation[12].
The KING closure technique is a method for closing perforations using a double-channel scope. It involves using an endoloop through one channel to encircle the defect and TTSCs through the second channel to secure the endoloop to the edges of the perforation. The endoloop is then tightened to close the defect[13,14].
The application of self-expandable metal stents (SEMS) has been documented in upper gastrointestinal perforations[15]. However, there are limited case reports on the use of SEMS in colorectal perforations, and the evidence supporting their practical application in this context is still in its early stages[2]. Furthermore, most instances where SEMS have been utilized to address perforations have been in the context of stent placement for a malignant stricture or perforations following the dilatation of a benign stricture[16].
Vacuum therapy, also known as "negative pressure wound therapy", has been a growing and professionally widespread procedure worldwide since the early 1990s. The new technique is fundamentally different from traditional passive wound management, which isolates the wound from the environment: The active wound management system provides a lower suction effect than the prevailing atmospheric pressure, thus facilitating and accelerating wound healing by continuously eliminating the effects that inhibit wound healing (secretions, toxins, etc.)[17]. This approach involves maintaining negative pressure to reduce edema, increase blood flow, decrease bacterial infection, and promote healing[18].
In 2004, Weidenhagen introduced the concept of endoluminal vacuum therapy and developed the Endolumical vacuum therapy (EndoVAC) technique to address anastomotic leaks following colorectal surgeries[19]. More recently, this approach has been utilized for managing iatrogenic colorectal perforations in multiple case reports[20,21]. The technique involves using a sponge cut to the size of the perforation or slightly smaller, as suction will naturally reduce the size of the perforation. This sponge is then attached to a nasogastric tube and placed in the perforation, where it shrinks gradually, starting to heal. The tube is secured by passing it through the gluteal cleft to one side of the hips and fixing it with sutures or adhesive dressing. It is important to note that this procedure requires sponge replacement every 3-5 days. For perforations less than 1 cm in size or those near the anal verge, patient movement should be limited to prevent sponge displacement. Mostly in those patients, the sponge needs to be replaced only twice until there is no contrast leak. Patients undergoing EndoVAC therapy should be kept nothing by mouth and receive total parenteral nutrition[22].
At present, there is a lack of large-scale studies on the use of EndoVAC in iatrogenic colorectal perforations, with the only available evidence coming from case reports[21]. However, several studies have discussed the success rates and median treatment duration using EndoVAC in patients with colorectal anastomotic leaks. The general median treatment duration for EndoVAC was 13 days, with a median of 3.5 sponge exchanges in a study from the Netherlands[23]. Abdalla et al[24]reported a 55% success rate, with a higher success rate (73%) when used as an initial treatment compared to a second-line treatment (33%). However, the median treatment duration in this study was 27 days, with a median of 6.6 sponge exchanges. A large meta-analysis conducted by Dhindsa et al[25], in 2021, reported a technical success rate of 99.86% and a clinical success rate of 84.99%. However, another meta-analysis reported a slightly lower clinical success rate at 81.4%[26].
The EndoVAC procedure has an achievable short learning curve and cost-effective outcomes. Expert endoscopists with prior experience in post-anastomosis follow-up endoscopies should have no trouble learning the technique after being involved in 5 to 10 cases. Also, the used accessories in EndoVAC are relatively inexpensive, and patients undergoing this procedure experience shorter hospital stays compared to those undergoing other treatment modalities such as surgery, which decreases the cost of the procedure[27].
The primary adverse events following EndoVAC treatment are typically stricture, which can generally be managed with endoscopic dilatation, and significant bleeding. However, these adverse events have been documented in cases involving the use of EndoVAC for upper gastrointestinal perforations[22].
Iatrogenic colonic perforations have been increasing lately due to the increase in therapeutic colonoscopic procedures and techniques. The current guidelines for the management of these complications need to be updated. The newly developed guidelines should address difficult situations of colonic perforations such as post-ESD delayed perforation as in coagulation syndrome. Also, the efficacy of SEMS for the management of perforations in the lower gastrointestinal perforations. In terms of EndoVAC, despite multiple case reports showing promising results, there is a lack of evidence such as controlled trials or at least prospective studies. There are still unanswered questions regarding EndoVAC, particularly concerning the necessary time interval between sponge exchanges. Additionally, manufacturing companies in the endoscopy field need to provide accessories specifically designed for this purpose. Finally we propose an algorithm for the management of iatrogenic colonic perforations based on the currently available evidence in literature (Figure 3).
| 1. | Lin CY, Pu TW. Colon perforation with severe peritonitis caused by erotic toy insertion and treated using vacuum-assisted closure: A case report. World J Clin Cases. 2024;12:3548-3554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Jung Y. Endoscopic Management of Iatrogenic Colon Perforation. Clin Endosc. 2020;53:29-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Lee J, Lee YJ, Seo JW, Kim ES, Kim SK, Jung MK, Heo J, Lee HS, Lee JS, Jang BI, Kim KO, Cho KB, Kim EY, Kim DJ, Chung YJ; Daegu-Gyeongbuk Gastrointestinal Study Group. Incidence of colonoscopy-related perforation and risk factors for poor outcomes: 3-year results from a prospective, multicenter registry (with videos). Surg Endosc. 2023;37:5865-5874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Cha RR, Kim HJ, Lee CM, Lee JM, Lee SS, Cho HJ, Ha CY, Kim HJ, Lee OJ. Clinical characteristics and outcome of iatrogenic colonic perforation related to diagnostic vs. therapeutic colonoscopy. Surg Endosc. 2022;36:5938-5946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kavic SM, Basson MD. Complications of endoscopy. Am J Surg. 2001;181:319-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 6. | Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21:10542-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | de'Angelis N, Di Saverio S, Chiara O, Sartelli M, Martínez-Pérez A, Patrizi F, Weber DG, Ansaloni L, Biffl W, Ben-Ishay O, Bala M, Brunetti F, Gaiani F, Abdalla S, Amiot A, Bahouth H, Bianchi G, Casanova D, Coccolini F, Coimbra R, de'Angelis GL, De Simone B, Fraga GP, Genova P, Ivatury R, Kashuk JL, Kirkpatrick AW, Le Baleur Y, Machado F, Machain GM, Maier RV, Chichom-Mefire A, Memeo R, Mesquita C, Salamea Molina JC, Mutignani M, Manzano-Núñez R, Ordoñez C, Peitzman AB, Pereira BM, Picetti E, Pisano M, Puyana JC, Rizoli S, Siddiqui M, Sobhani I, Ten Broek RP, Zorcolo L, Carra MC, Kluger Y, Catena F. 2017 WSES guidelines for the management of iatrogenic colonoscopy perforation. World J Emerg Surg. 2018;13:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Ramage JI, Grisolano SW, Petersen BT, Schroeder KW, Ott BJ, Gostout CJ. Risk Factors Associated with Colonoscopic Perforation. Gastrointest Endosc. 2004;59:P135. [DOI] [Full Text] |
| 9. | Paspatis GA, Arvanitakis M, Dumonceau JM, Barthet M, Saunders B, Turino SY, Dhillon A, Fragaki M, Gonzalez JM, Repici A, van Wanrooij RLJ, van Hooft JE. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement - Update 2020. Endoscopy. 2020;52:792-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1833] [Article Influence: 122.2] [Reference Citation Analysis (1)] |
| 11. | Lee JH, Kedia P, Stavropoulos SN, Carr-Locke D. AGA Clinical Practice Update on Endoscopic Management of Perforations in Gastrointestinal Tract: Expert Review. Clin Gastroenterol Hepatol. 2021;19:2252-2261.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Han JH, Park S, Youn S. Endoscopic closure of colon perforation with band ligation; salvage technique after endoclip failure. Clin Gastroenterol Hepatol. 2011;9:e54-e55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ryska O, Martinek J, Filipkova T, Dolezel R, Juhasova J, Motlik J, Zavoral M, Ryska M. Single loop-and-clips technique (KING closure) for gastrotomy closure after transgastric ovariectomy: a survival experiment. Wideochir Inne Tech Maloinwazyjne. 2012;7:233-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ryu JY, Park BK, Kim WS, Kim K, Lee JY, Kim Y, Park JY, Kim BJ, Kim JW, Choi CH. Endoscopic closure of iatrogenic colon perforation using dual-channel endoscope with an endoloop and clips: methods and feasibility data (with videos). Surg Endosc. 2019;33:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Raju GS. Endoscopic closure of gastrointestinal leaks. Am J Gastroenterol. 2009;104:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Kim SW, Lee WH, Kim JS, Lee HN, Kim SJ, Lee SJ. Successful management of colonic perforation with a covered metal stent. Korean J Intern Med. 2013;28:715-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1526] [Cited by in RCA: 1442] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 18. | Genecov DG, Schneider AM, Morykwas MJ, Parker D, White WL, Argenta LC. A controlled subatmospheric pressure dressing increases the rate of skin graft donor site reepithelialization. Ann Plast Surg. 1998;40:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Vignali A, De Nardi P. Endoluminal vacuum-assisted therapy to treat rectal anastomotic leakage: A critical analysis. World J Gastroenterol. 2022;28:1394-1404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (3)] |
| 20. | Slater NR, Loomes DE. Endoscopic Vacuum-Assisted Wound Closure of a Rectal Perforation in the Setting of Active Ulcerative Colitis. Am J Gastroenterol. 2021;116:1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Zumblick M, Stathopoulos P, Gress TM, Denzer UW. Endoscopic Vacuum Therapy for Iatrogenic Rectal Perforation. Case Rep Gastroenterol. 2022;16:425-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Wikiel KJ, Mcconnell B, Bollinger D, Abbitt D, Ahrendt SA, Mccarter MD, Morton AP, Pierraci FM, Jones EL. Endoluminal wound vacuum therapy for gastrointestinal leaks: current state and future directions. Ann Laparosc Endosc Surg. 2023;8:19-19. [DOI] [Full Text] |
| 23. | Borstlap WAA, Musters GD, Stassen LPS, van Westreenen HL, Hess D, van Dieren S, Festen S, van der Zaag EJ, Tanis PJ, Bemelman WA. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc. 2018;32:315-327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Abdalla S, Cotte E, Epin A, Karoui M, Lefevre JH, Berger A, Marchal F, Denost Q, Penna C, Benoist S, Brouquet A; on behalf the French GRECCAR group. Short-term and Long-term Outcome of Endoluminal Vacuum Therapy for Colorectal or Coloanal Anastomotic Leakage: Results of a Nationwide Multicenter Cohort Study From the French GRECCAR Group. Dis Colon Rectum. 2020;63:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Dhindsa BS, Naga Y, Saghir SM, Daid SGS, Chandan S, Mashiana H, Dhaliwal A, Sidhu A, Sayles H, Ramai D, Bhat I, Singh S, McDonough S, Adler DG. Endo-sponge in management of anastomotic colorectal leaks: a systematic review and meta-analysis. Endosc Int Open. 2021;9:E1342-E1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kühn F, Schardey J, Wirth U, Schiergens T, Crispin A, Beger N, Andrade D, Drefs M, Zimmermann P, Burian M, Andrassy J, Werner J. Endoscopic vacuum therapy for the treatment of colorectal leaks - a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Ward MA, Hassan T, Burdick JS, Leeds SG. Endoscopic vacuum assisted wound closure (EVAC) device to treat esophageal and gastric leaks: assessing time to proficiency and cost. Surg Endosc. 2019;33:3970-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |