INTRODUCTION
Chronic osteomyelitis, a disease causing substantial morbidity and mortality, is a major challenge in orthopedic surgery. In severe open fractures, one of the most devastating complications is infection, which can lead to nonunion and long-lasting sinus fractures[1]. Achieving remission is notoriously difficult, with some studies reporting failure rates of 20%-60%[2,3]. The conventional treatment strategy for chronic osteomyelitis requires radical debridement, and all dead and infected tissues should be removed[4]; however, thorough debridement often results in large bone defects, which remains a challenge for most orthopedic surgeons. A large bone defect is defined as a defect exceeding 2-2,5 times the diameter of the affected bone[5]. Various techniques have been used to reconstruct bone defects, such as segmental bone transport, transfer of the vascular fibula, autogenous bone graft, and megaprothesis[6]. In this case, the induced-membrane technique, which was first reported by Masquelet and Begue in 1986[7] and described as a two-stage reconstruction of bone defects, was used to solve this problem. The induced-membrane technique is a widely used method supported by numerous publications with good outcomes and a high success rate. Here, in contrast to the classical Masquelet technique, we used allogeneic cortical bone to reconstruct a bone defect with a radius of 9 cm.
CASE PRESENTATION
Chief complaints
A sinus was formed in the left forearm which discharged pus for 6 months.
History of present illness
The patient was a 40-year-old truck driver, when after a car accident in 2021, he was treated at another hospital by successive reduction of the left radius Gustilo II open fracture and osteosynthesis with external fixation and insertion of a locking compression plate (LCP). One week after the internal fixation surgery, the patient developed a local infection and underwent two rounds of debridement within one month. The debridement surgeries failed, and a sinus formed, which occasionally discharged pus.
History of past illness
The patient had been in good health.
Personal and family history
The patient was married, his wife and 2 children were in good health; the patient did not smoke or drink alcohol.
Physical examination
The patient had high skin temperature and dysfunction on the left forearm.
Laboratory examinations
The tissue culture results of the patient’s sinus showed methicillin-resistant Staphylococcus aureus (MRSA) infection; all of the infection indicators, including the neutrophil level, C-reactive protein, erythrocyte sedimentation rate, and procalcitonin level, were negative.
Imaging examinations
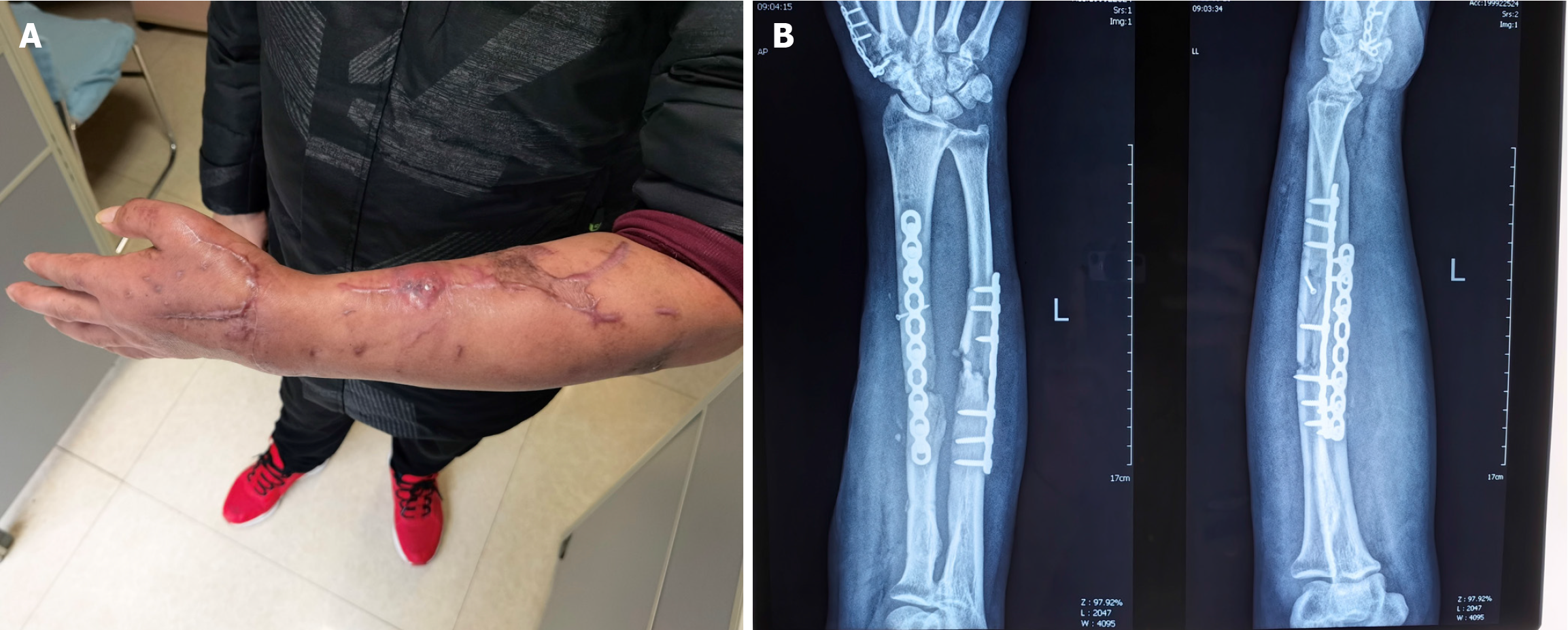
The patient’s X-ray showed nonunion in the middle of his left radius (Figure 1).
Figure 1 Presentation of the patient.
A: Skin sinus of the patient; B: Preoperative X-ray showing the infectious nonunion of the left radius.
FINAL DIAGNOSIS
According to the patient’ complaints, history of past illness, physical examination, laboratory examinations; and imaging examinations, his final diagnosis was a type IIIA[8] chronic osteomyelitis of the left radius.
TREATMENT
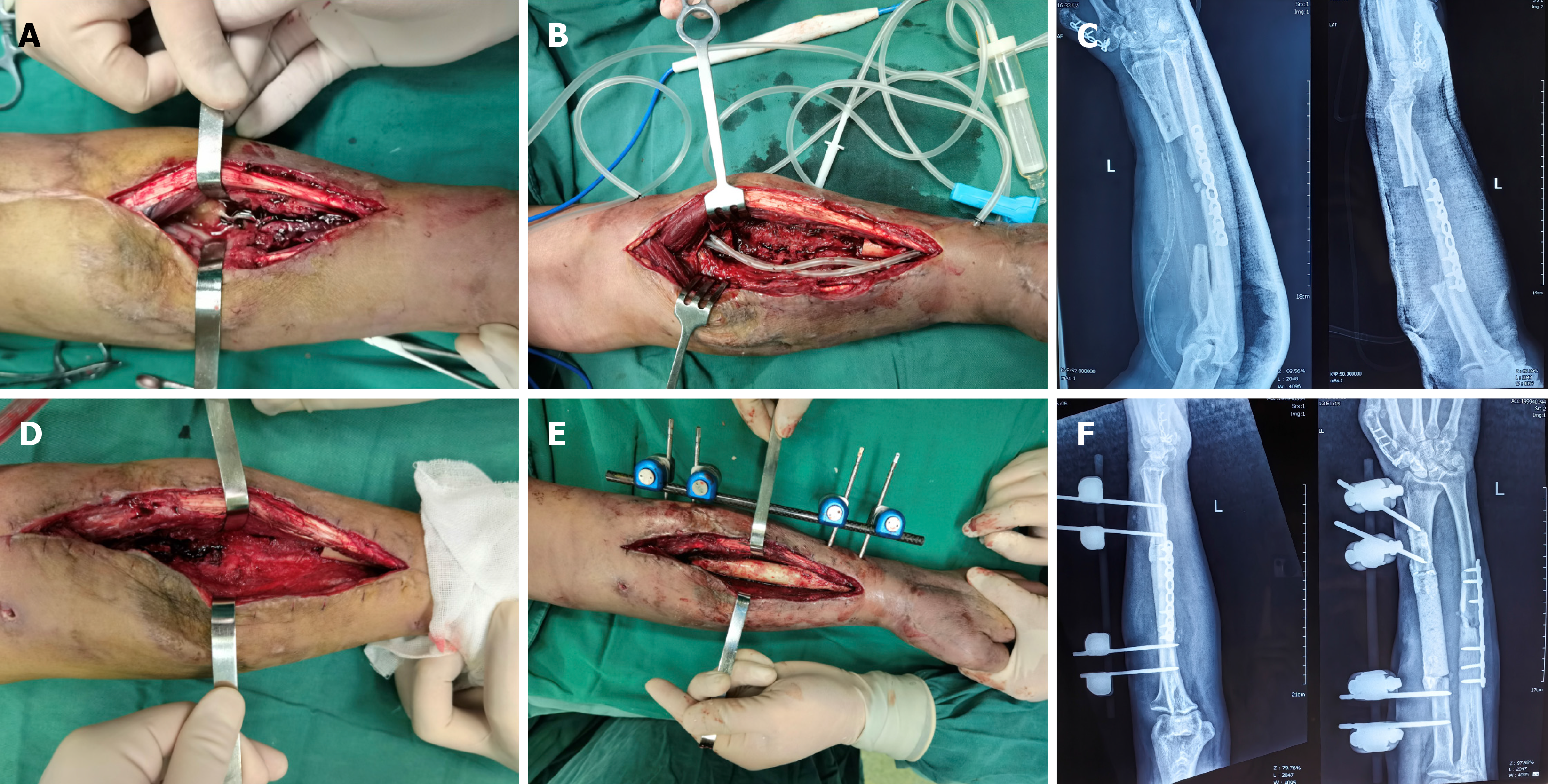
We proposed a two-stage reconstructive treatment to the patient. The first surgical step was characterized by the removal of the implant plate and the infected bone, and complete debridement was performed: The bone and wound were removed until punctate bleeding occurred[9]. Drainage tubes and vacuum sealing drainage (VSD) tubes were used (Figure 2A-C). After 2 weeks of VSD treatment and three consecutive negative tissue culture results, the bone defect was filled with an antibiotic (vancomycin) cement spacer, and the clavicle was stabilized with an external fixator (Figure 2D-F).
Figure 2 Debridement and cement implantation to the patient.
A: Infectious focus of the left radius; B: Removal of the implant and infectious bone segment; and placement of drainage tubes and vacuum sealing drainage; C: X-ray after debridement; D: Effective resolution of the focus; E: Placement of the antibiotic spacer and external fixation; F: X-ray after placement of the antibiotic spacer and external fixation.
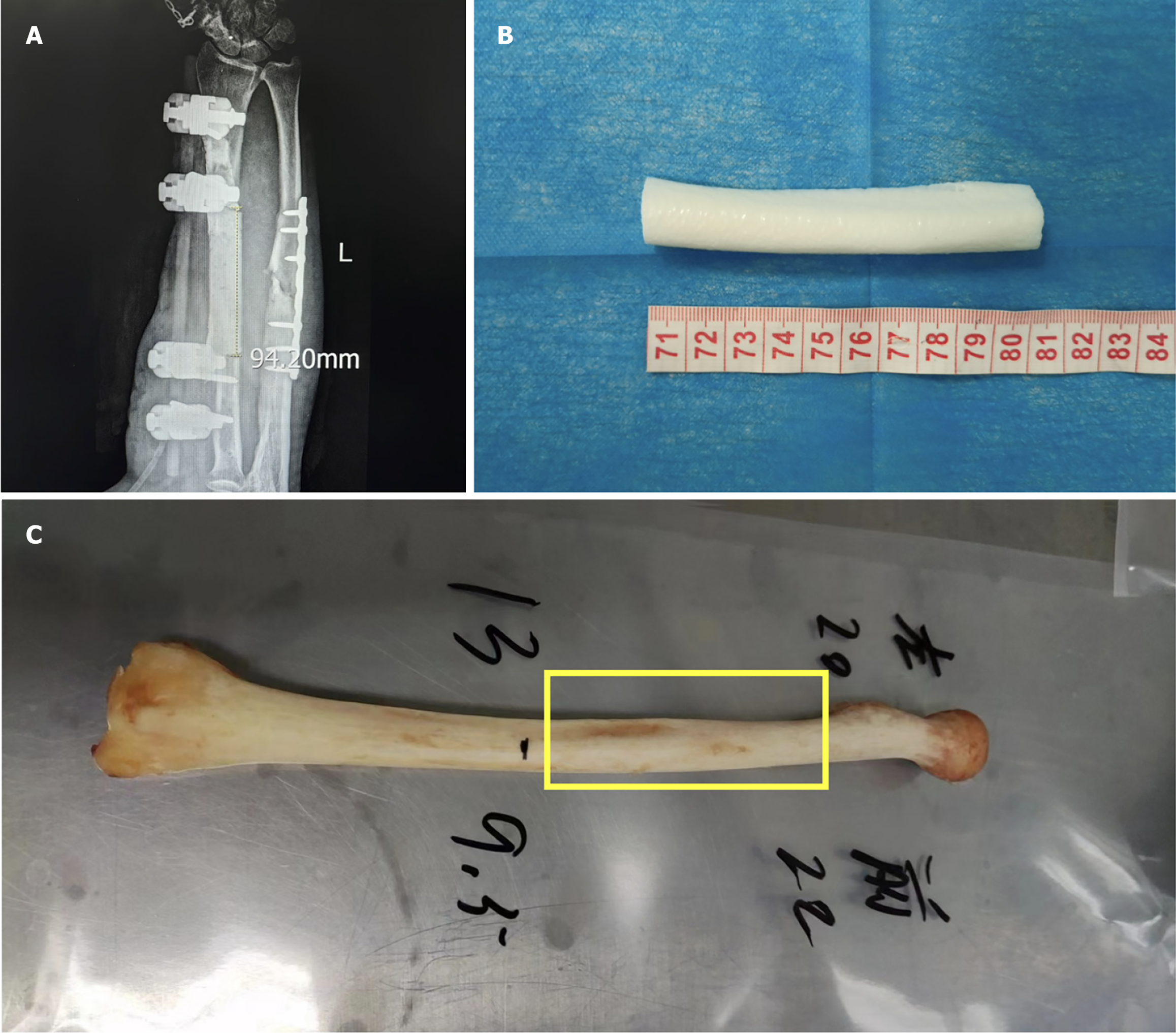
A bone defect of greater than 9 cm was measured through the imaging system (Figure 3A). The nonunion scoring system[10,11] score was 68 points, which indicated a high possibility of bone nonunion. The model of the defect radius segment was restored via mirror 3D-printing technology (Figure 3B), and by comparison with a local bone bank, the donor bone segment was identified (Figure 3C).
Figure 3 Bone defect and selection of the donor bone segment.
A: Length of the left radius defect; B: 3D-printed model of the defective bone segment; C: Donor bone graft matched to the defective radius.
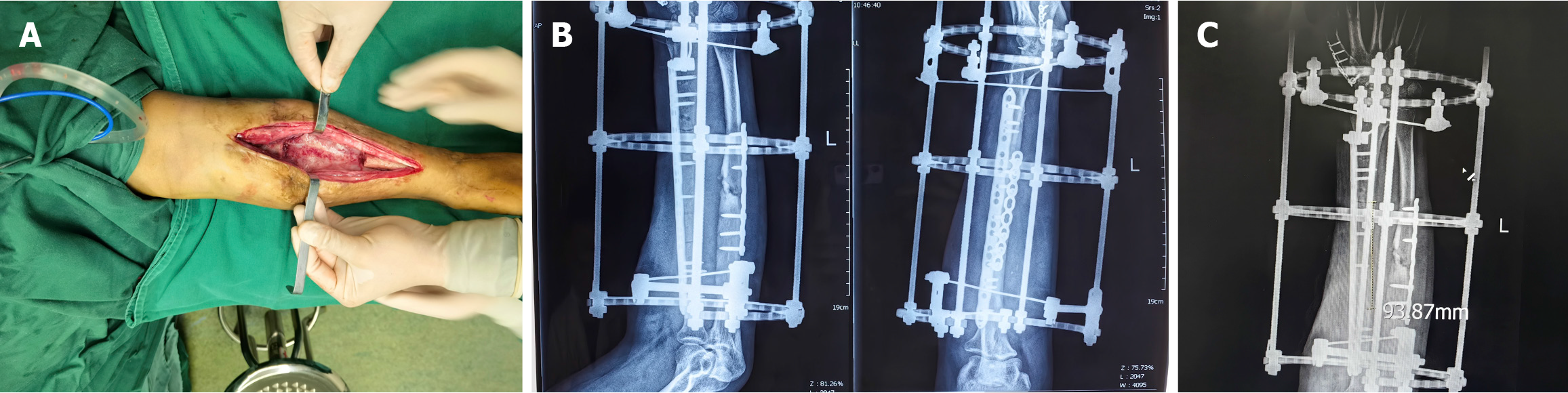
After 12 weeks, we performed the second surgical step and removed the cement spacer and external fixator. The induced membrane (Figure 4A) was well protected, and the bone defect was filled with a large allogeneic cortical bone graft. The stability of the bone was maintained through an LCP and Ilizarov external fixator (Figure 4B). X-ray examination revealed that the length of the graft was consistent with that of the bone defect measured preoperatively (Figure 4C).
Figure 4 The induced membrane and X-ray after bone implantation.
A: Cavity and induced membrane after spacer removal; B: Postoperative X-ray; C: Length of the allogeneic cortical bone graft.
OUTCOME AND FOLLOW-UP
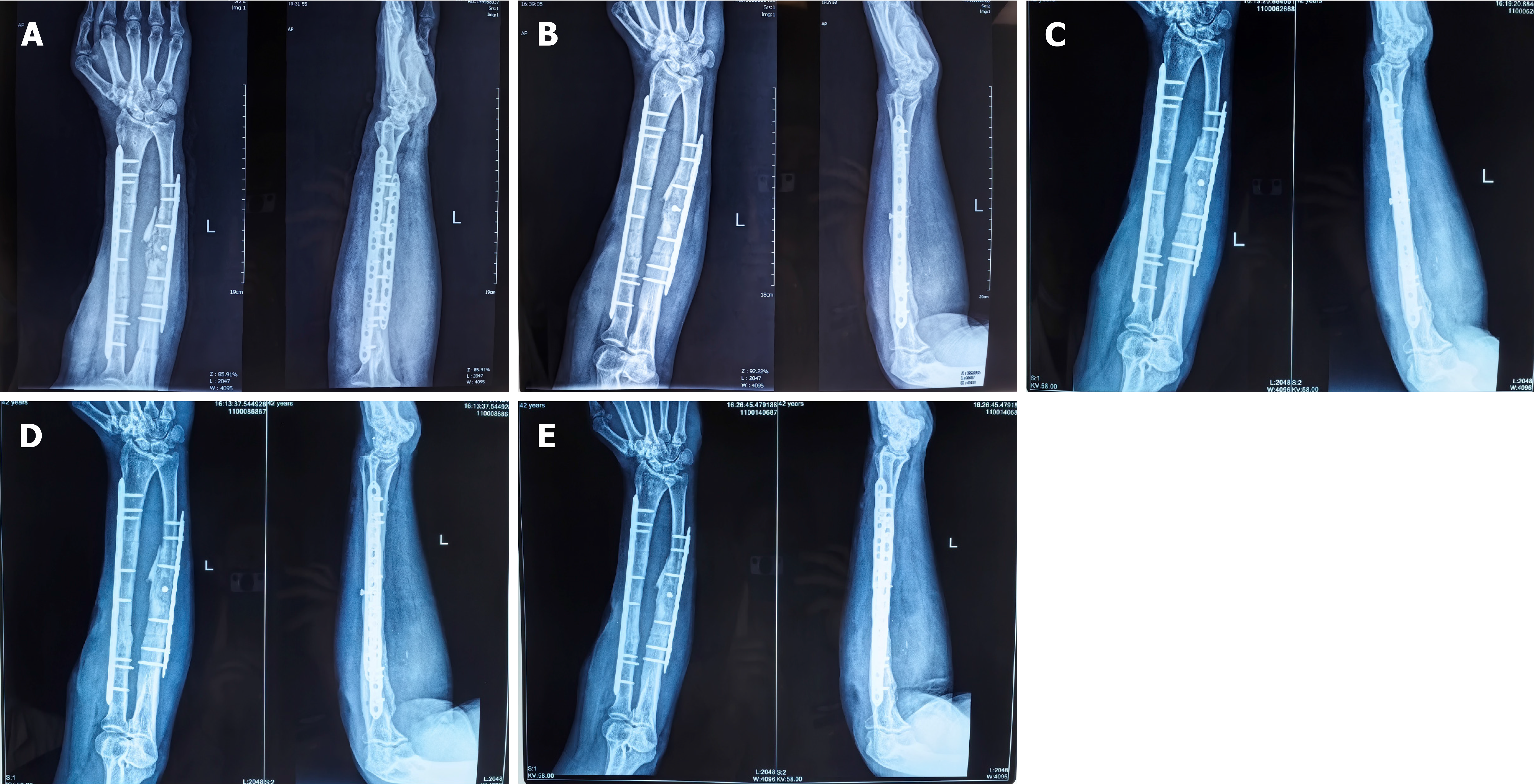
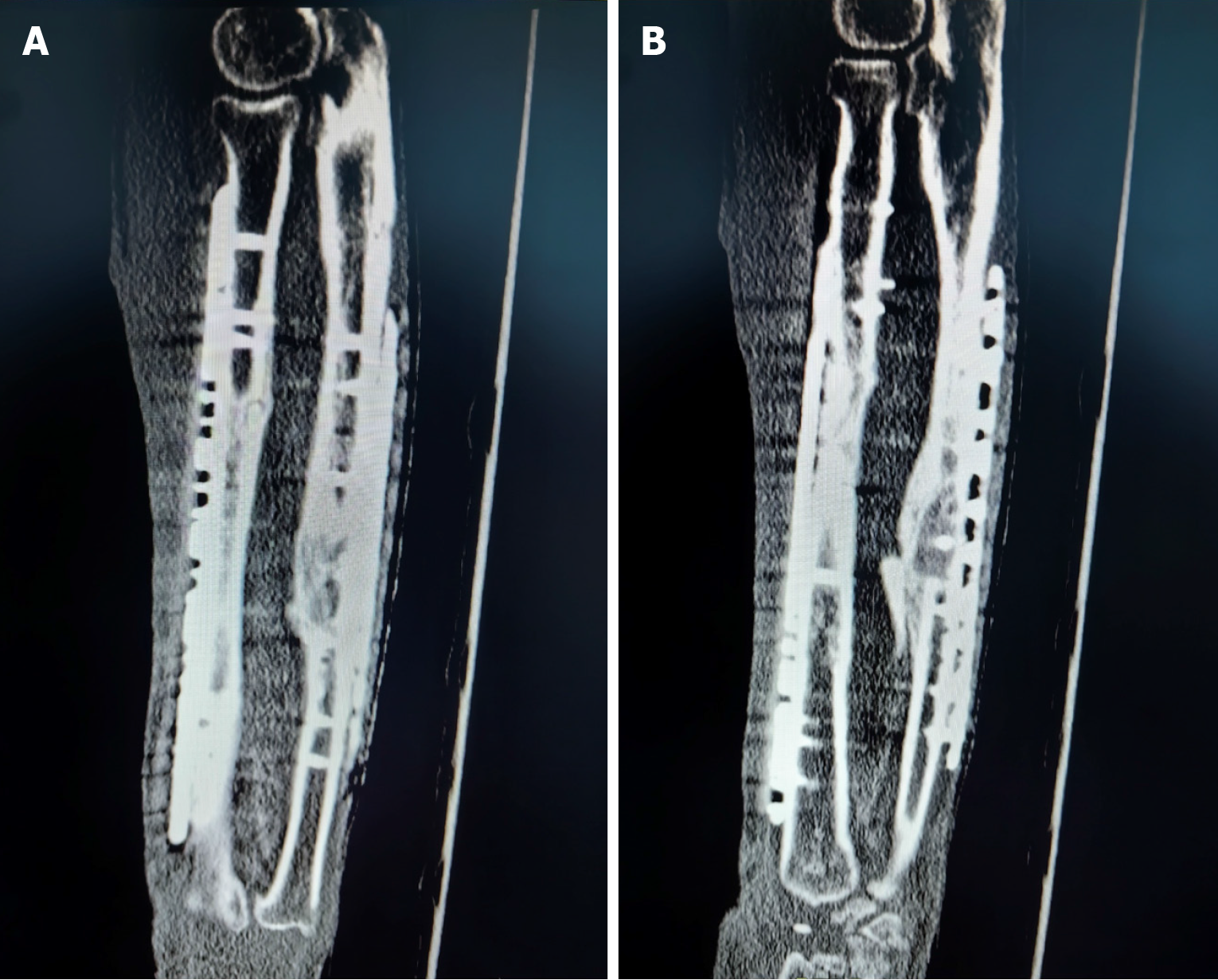
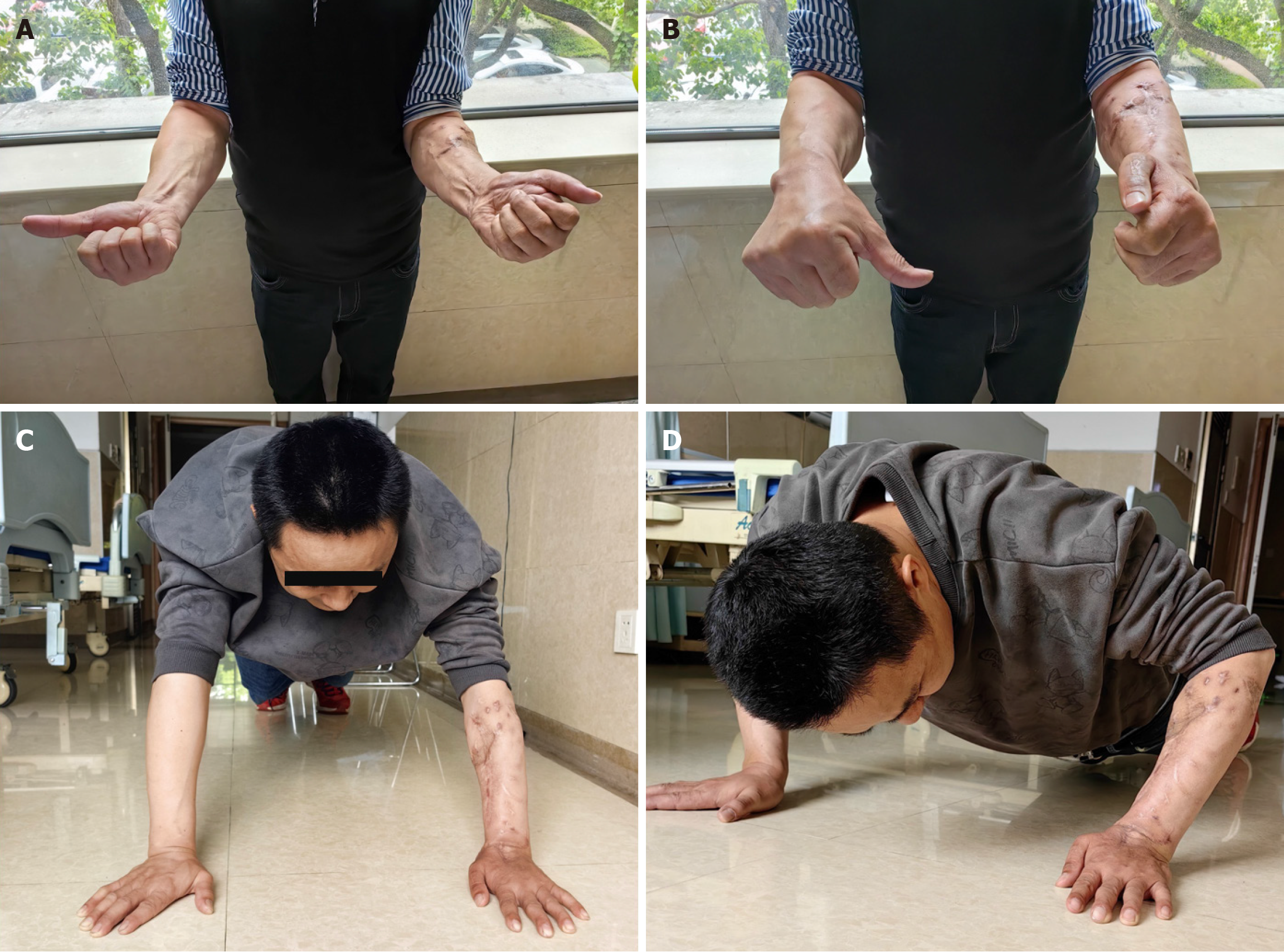
The Ilizarov external fixator was removed at 3 months postoperatively, and callus formation was observed in the radiographs (Figure 5A). Radiological and clinical follow-up was carried out at 6, 10, 14 and 23 months (Figure 5B-E), and no complications developed during the postoperative period. The patient returned to his truck-driving activities without pain at 10 months after the surgery, and osseous healing was noted at the 14-month follow-up through computed tomography scanning (Figure 6). The 2-year postoperative follow-up results showed that good function and bone strength were achieved (Figure 7).
Figure 5 Radiographs at follow-up.
A: X-ray image after 3 months; B: X-ray image after 6 months; C: X-ray image after 10 months; D: X-ray image after 14 months; E: X-ray image after 24 months.
Figure 6 Healing of the bone graft.
A: Computed tomography (CT) scan after 14 months, demonstrating proximal healing of the allogenic cortical bone graft; B: CT scan after 14 months, demonstrating distal healing of the allogenic cortical bone graft.
Figure 7 Functional recovery of the patient at 2 years follow-up.
A: Supination function of the forearm; B: pronation function of the forearm; C: The support function of the forearm; D: Strength of the forearm.
DISCUSSION
In trauma, one of the most devastating complications of open fractures is infection, which is a primary mechanism for fracture nonunion[1]. In the present case, the patient had undergone two failed debridement procedures and a long course. Consequently, we established a more aggressive strategy: Removal of the entire infected bone segment as well as the implant. Among all the strategies for large bone defect reconstruction, we chose the Masquelet technique, which was initially described by Masquelet and Begue[7] in 1986 as a treatment for tibia nonunion, to manage the patient’s bone defects greater than 9 cm in length[12]. After years of refinement, this technique has become an effective method for managing bone defects and is supported by numerous publications reporting very good outcomes and a high success rate[13]. The procedure consists of two stages[7]. In the first stage, polymethyl methacrylate cement is implanted in the zone of the bone defect. The cement acts as a spacer that maintains the bone length and facilitates the formation of a membrane that contains osteoprogenitor cells and releases inductive molecules that stimulate osteogenesis[14,15]. The second stage is initiated after 6 to 8 weeks, when the secretion of growth factors reaches its maximum level and sufficient vascularization of the induced membrane occurs[14]. The membrane is then opened, the cement is removed, and the defect area is recommended to be filled with autogenous cancellous bone graft harvested from the iliac crest. The induced membrane left in place prevents the resorption of the graft and promotes bone mineralization. In our case, the second stage was performed after 12 weeks because of the effects of coronavirus disease 2019 pandemic, which was acceptable.
In the application of classical Masquelet technology, a bone substitute such as a demineralized allograft can be added to an insufficient amount of autograft at a ratio that does not exceed 1:3[14]. Many researchers have reported innovations in bone graft methods. Most of them have reported the application of an autologous bone graft alone or an enriched bone graft [autologous bone graft with allograft, bone morphogenetic protein (BMPs), osteoprogenitor cells, growth factors, platelet-rich plasma, and bone marrow concentrate aspirate][16-24]. The main effect of bone substitute materials is filling the cavity[25], its impact on bone healing remains to be further studied. Additionally, bone-induced proteins such as BMPs, its effect to bone healing remains unclear. The use of the Masquelet technique with a pure allograft is very rare. Issaoui et al[26] reported a modified Masquelet technique using allogeneic cancellous bone graft only for an 8 cm bone defect of the femur. Ramos et al[27] reported the use of a fibular strut allograft for segmental femur defects. In our case, the patient strongly resisted autogenous iliac bone grafting. Moreover, the exact mechanism of the Masquelet technique remains unclear. Considering that the function of the induced membrane includes recruiting mesenchymal stem cells, which differentiate into osteoblasts, release inductive molecules that stimulate osteogenesis, and thereby prevent resorption of the graft and promote bone mineralization[14], we believed that a pure allograft of cortical bone could be mineralized completely during stage II of the Masquelet technique. A bone callus started to form at 12 weeks after the second stage, and complete bony union was observed at 14 months of follow-up. This delay of consolidation is acceptable and consistent with the literature[16,28]. Masquelet et al[29] recommended a high initial rigidity of fixation to promote incorporation, followed by more flexible fixation to promote bone remodeling and mineralization. In our case, we chose initial internal fixation with an Ilizarov external fixator to assure the absolute stability of the fracture. To optimize the quality of the induced membrane, the strength of a cortical allograft would allow early mobility, which could provide better functional rehabilitation. With complete bone union, the 2-year follow-up results confirmed that the modified Masquelet technique guarantees the growth of a newly formed bone of good quality, highlighting that the exclusive application of allograft cortical bone is compatible with this technique.
To improve the probability of fracture healing, we adopted the bone fragmentation technique. As its name implies, the broken end of the bone was fragmented to increase the contact area. However, this technique did not seem to apply to a large allograft cortical bone because decalcification makes the bone graft more brittle, resulting in an uncontrollable fracture, which may prolong the fracture-healing period (Figure 6A).
CONCLUSION
The Masquelet technique is a simple, reliable, and reproducible technique that can be used to reconstruct large bone defects. In our case, the exclusive use of allograft cortical bone was as successful as the use of an autologous bone graft. With the advent of bone banks, it is possible to obtain an unlimited amount of allograft. The reason for this success of the present allograft and the exact osteogenic mechanism of the Masquelet technique remain unclear. Thus, additional research and large studies are necessary before giving recommendations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Zhou X S-Editor: Liu H L-Editor: A P-Editor: Zhang XD