Published online Feb 16, 2025. doi: 10.12998/wjcc.v13.i5.97629
Revised: October 11, 2024
Accepted: November 4, 2024
Published online: February 16, 2025
Processing time: 167 Days and 23.4 Hours
Congenital cytomegalovirus (CMV) infection represents a significant public health concern as the most prevalent viral infection in newborns, potentially leading to severe neurological and developmental complications. The majority of cases are asymptomatic and remain undetected during pregnancy due to the absence of effective screening methods.
A 27-year-old primigravida presented for early pregnancy ultrasound, which revealed an atypical finding: A normal anechoic thalamus appearing hyperechoic on the mid-sagittal view of the fetal head. Subsequent ultrasound examinations during mid and late gestation demonstrated classic intracranial features sug
Congenital CMV infection occurs during the first trimester may manifest as hyperechoic thalamus which can be revealed by ultrasound in the mid-saggital view of the fetal head. Future research should investigate the correlation between echogenic thalamus and developmental outcomes, as well as explore early sc
Core Tip: We present a case of a fetus infected with cytomegalovirus, characterized by distinctive ultrasound findings during early gestation. The patient underwent a thorough diagnostic evaluation, systematically excluding chromosomal ab
- Citation: Chen XL, Zhang LQ, Bai LL. Ultrasound features of congenital cytomegalovirus infection in the first trimester: A case report. World J Clin Cases 2025; 13(5): 97629
- URL: https://www.wjgnet.com/2307-8960/full/v13/i5/97629.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i5.97629
Cytomegalovirus (CMV) is a neurotropic virus and the leading viral cause of intrauterine infection, affecting approximately 0.7% of live neonates worldwide[1]. Major affected neonates will be asymptomatic at birth, and more than one fourth of infected children will have long-term sequelae, including sensorineural hearing loss and neurological impairments[2], when the infection happens in the first trimester of pregnancy[3]. Congenital CMV-infected fetuses may be categorized into three groups: Asymptomatic, mild to moderate symptomatic, and severely symptomatic, with out
Owing to the limited effectiveness of screening tests, routine prenatal screening for CMV infection during pregnancy is not implemented in most countries globally[5]. Consequently, routine ultrasound examinations during pregnancy assume a crucial role in the prenatal diagnosis of CMV infection. However, prenatally detecting fetuses that are asym
A 27-year-old nulliparous woman presented at our hospital for a routine ultrasound examination.
The patient was healthy, and the pregnancy was uneventful.
The patient had no past illness.
Both the pregnant woman and her husband had no significant personal or family history.
The physical examinations of the pregnant woman did not reveal any abnormalities.
Prenatal aneuploidy screening indicated a high risk for trisomy 21. Subsequently, amniocentesis was performed at 18 weeks of gestation (GA), with both karyotype and chromosomal microarray analyses yielding normal results. A to
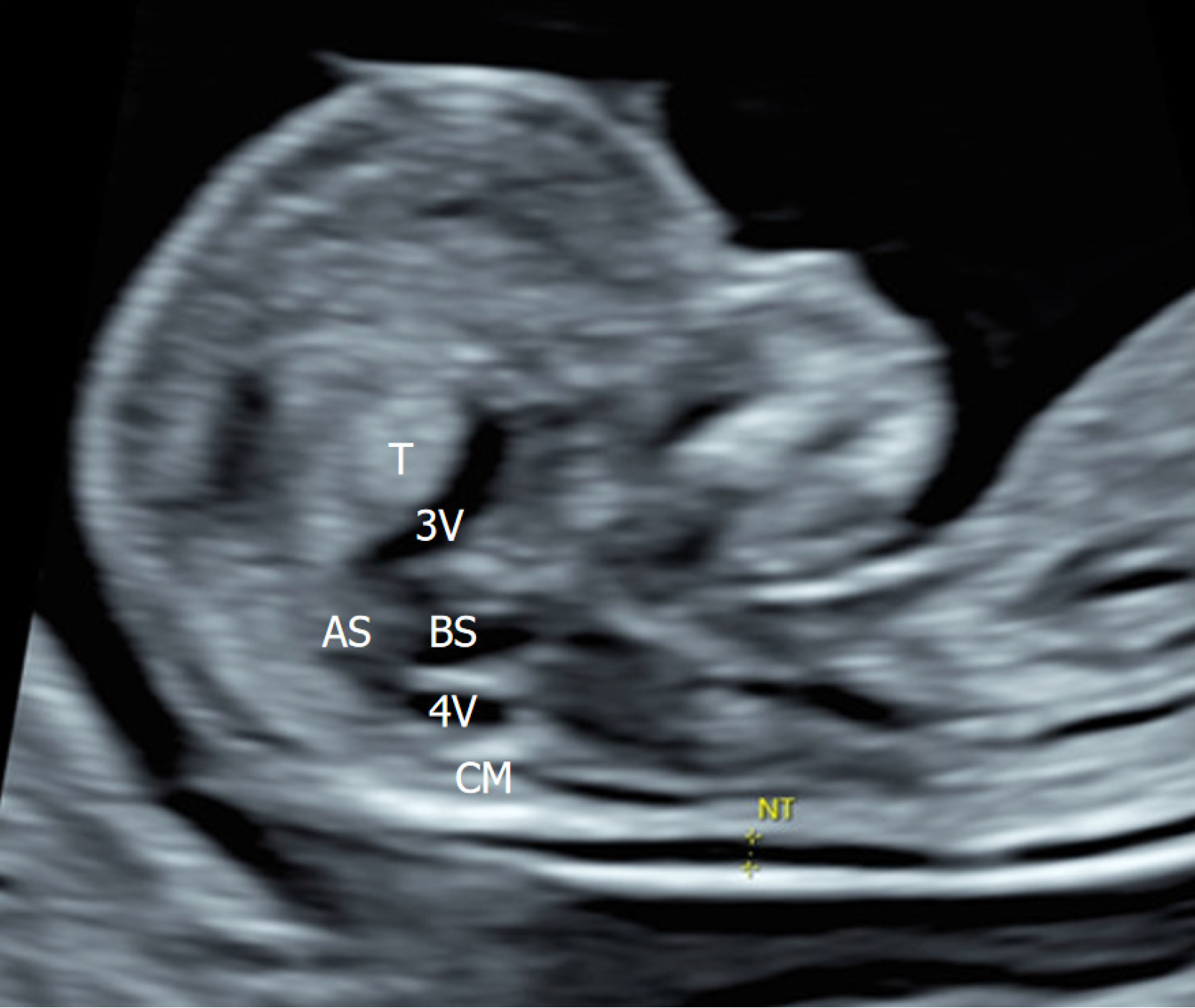
The routine ultrasound examination at 12 weeks of GA showed a normal nuchal translucency of 1.0 mm and a crown-rump length of 59 mm, which is consistent with the expected gestational age. The ultrasound assessment was performed in accordance with the International Society of Ultrasound in Obstetrics and Gynecology guidelines[9] using a Voluson™ E10 machine (GE Healthcare) with C2-9-D probe, operating at a frequency range of 2-9 MHz. By aligning the ultrasound beam with the mid-sagittal suture from the anterior fontanel perspective, a mid-sagittal view of the fetal brain is obtained. This view shows several midline cerebral structures, including the nasal bone, third ventricle, thalamus, brainstem, fourth ventricle, cisterna magna, aqueduct of Sylvius, and nuchal translucency. The anatomical examination was mostly normal, except for a round hyperechoic thalamus located above the non-obliterated third ventricle (Figure 1), which usually appears anechoic. A subsequent examination at 23 weeks of GA indicated that the fetus was small for gestational age and exhibited microcephaly and occipital calcification. The couple declined a repeat amniocentesis for CMV DNA detection. Follow-up ultrasound examinations at 30, 32, 35, and 38 weeks of GA revealed progressive manifestations of mic
The diagnosis of prenatal CMV infection was established based on the presence of characteristic intracranial features, clinical manifestations, serological findings for CMV-IgG and IgM, and normal karyotype and microarray results, while ruling out other potential infections.
The infant did not receive any specific treatment due to the negative result of the CMV-DNA urine test. Only supportive care measures were administered.
At six months of age, the infant received a diagnosis of epilepsy and developmental delays. Subsequently, she com
The gold standard for diagnosing CMV involves detecting its DNA in amniotic fluid using polymerase chain reaction prenatally, or in urine or saliva within the first three weeks of life. However, Blázquez-Gamero et al[11] reported a case where CMV DNA was detected in the amniotic fluid. The patient received a dose of hyperimmune globulin for therapy after amniocentesis, but this treatment was later proven ineffective in a randomized controlled trial[12], and subsequently became negative in urine after birth. This scenario might be applicable to our case. IgG antibodies in an infected fetus can persist until the age of 2 years[13], while IgM typically lasts only a few months. Therefore, maternal CMV-IgM negativity does not preclude amniotic fluid sampling for CMV-DNA-polymerase chain reaction analysis when CMV infection is suspected[8]. These findings align with the serological results observed at 6 months in this case.
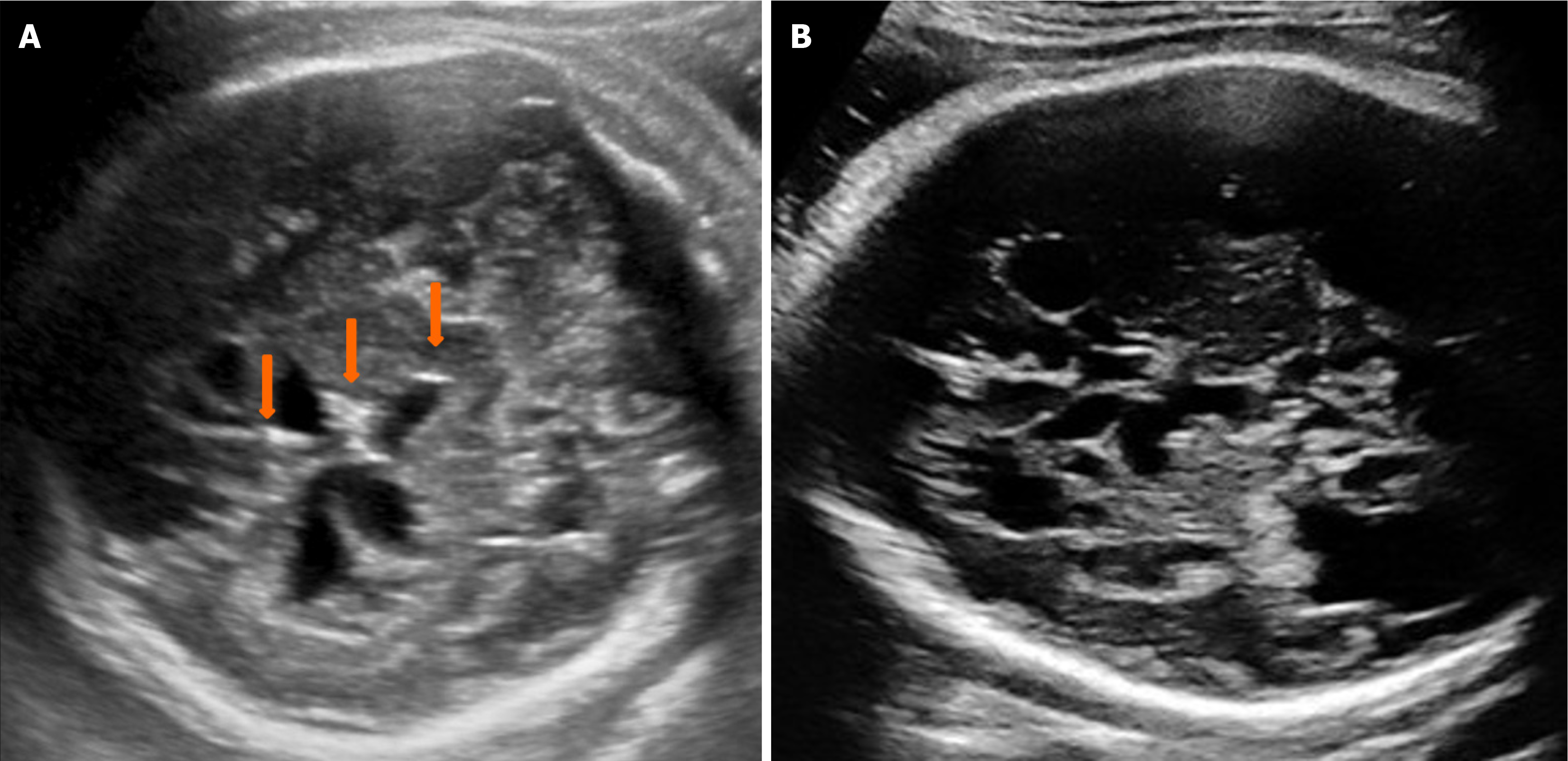
Definitive diagnosis of fetal infection typically requires invasive testing, primarily amniocentesis, performed 6-8 weeks after the initial maternal infection. Amniocentesis is generally delayed until 18-20 weeks of GA, as fetal urination is not sufficiently developed before this stage. Consequently, diagnosing fetal CMV infection during the first trimester remains challenging. However, the ultrasound manifestations of CMV infection during the second and third trimesters of pregnancy have been extensively documented in the medical literature[4,6,14]. In our particular case, we observed and documented the typical intracranial features indicative of CMV infection, which included microcephaly (below two standard deviations), ventriculomegaly, lissencephaly, intracranial multiple cysts and calcifications. In our experience, typical intracranial findings of CMV infection - usually two or more - can often be confirmed virologically. This ob
This case provides significant insights into the complex dynamics of CMV infection affecting the fetal brain during the critical first trimester of pregnancy. It suggests that the thalamus may be the initial brain region impacted by CMV infection, emphasizing its vulnerability during early development. The study meticulously delineates the midsagittal anatomical structures of both the thalamus and the third ventricle within the fetal head during the first trimester, as referenced in previous works[16,17]. Specifically, it describes the thalamus as a round, hyperechoic structure situated cephalically, while the third ventricle is characterized by its slit-like, anechoic appearance located caudally. Clinically, this may indicate a need for invasive assessment due to an unidentified irregularity that has not been fully understood or diagnosed. This raises concerns among medical professionals about its implications for patient care and the need for further investigation into this anomaly. To our knowledge, this research is the first comprehensive study to accurately depict the anatomical structures of the third ventricle and thalamus during the first trimester. This is particularly important as previous pathological studies have often reported the third ventricle as obliterated[17].
Several researchers have documented various thalamic hyperechogenicities[8,18], some associated with CMV infection and others not. Bronshtein et al[18] reported seven cases of isolated hyperechogenic foci in the fetal thalamus at 14-16 weeks of GA; six fetuses exhibited one hyperechogenic focus, while one presented two foci. These foci ranged in size from 2 mm to 4 mm. In all instances, serologic work-up and karyotype were normal, the hyperechogenic foci resolved by mid-pregnancy, and all fetuses were normal at delivery, remaining healthy during follow-up until nine years of age. They concluded that isolated hyperechogenic foci in the thalamic region in early pregnancy are probably benign in nature. In contrast to previous cases, this instance of thalamic hyperechogenicity was detected at 12 weeks of GA, manifesting as a round hyperechoic thalamus. While this finding disappeared in the mid-trimester, other intracranial signs of CMV infection emerged. Our observations during the first trimester of pregnancy provided a novel, non-invasive approach that potentially facilitates early diagnosis of fetal CMV infection, which is essential for safeguarding fetal health and de
A multicenter study showed that fetal brain magnetic resonance imaging (MRI) can detect approximately 10.5% additional anomalies in fetuses with CMV infection and normal neurosonography[19]. However, both fetal and neonate MRI did not reveal more information compared with fetal neurosonography in our case. This may be attributed to a lack of awareness about CMV infection among radiologists and inadequate training of technologists in performing advanced fetal MRI[20]. Fetal MRI is not recommended prior to 18 weeks of GA because it does not usually provide additional information than ultrasound examinations[20].
This study presents potential limitations. This case report examines a unique instance of a fetus exhibiting classic ultrasound findings indicative of CMV infection, emphasizing the critical importance of prompt virological diagnosis. However, due to the absence of immediate viral testing, a definitive virological diagnosis was not established, high
CMV infection, especially when occurring during the critical first trimester of pregnancy, may manifest as a hyperechoic thalamus. This condition can be effectively visualized through ultrasound imaging in the mid-sagittal view of the fetal head, offering crucial insights into the developing brain. Future research should focus on thoroughly investigating the complex relationship between the presence of an echogenic thalamus and various developmental outcomes in affected infants. Additionally, exploring innovative early screening methods for suspected congenital CMV infection could significantly improve early diagnosis and intervention strategies.
| 1. | Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 863] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 2. | Korndewal MJ, Oudesluys-Murphy AM, Kroes ACM, van der Sande MAB, de Melker HE, Vossen ACTM. Long-term impairment attributable to congenital cytomegalovirus infection: a retrospective cohort study. Dev Med Child Neurol. 2017;59:1261-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Faure-Bardon V, Magny JF, Parodi M, Couderc S, Garcia P, Maillotte AM, Benard M, Pinquier D, Astruc D, Patural H, Pladys P, Parat S, Guillois B, Garenne A, Bussières L, Guilleminot T, Stirnemann J, Ghout I, Ville Y, Leruez-Ville M. Sequelae of Congenital Cytomegalovirus Following Maternal Primary Infections Are Limited to Those Acquired in the First Trimester of Pregnancy. Clin Infect Dis. 2019;69:1526-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 4. | Khalil A, Sotiriadis A, Chaoui R, da Silva Costa F, D'Antonio F, Heath PT, Jones C, Malinger G, Odibo A, Prefumo F, Salomon LJ, Wood S, Ville Y. ISUOG Practice Guidelines: role of ultrasound in congenital infection. Ultrasound Obstet Gynecol. 2020;56:128-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Walker SP, Palma-Dias R, Wood EM, Shekleton P, Giles ML. Cytomegalovirus in pregnancy: to screen or not to screen. BMC Pregnancy Childbirth. 2013;13:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Leruez-Ville M, Ville Y. Fetal cytomegalovirus infection. Best Pract Res Clin Obstet Gynaecol. 2017;38:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Malinger G, Lev D, Zahalka N, Ben Aroia Z, Watemberg N, Kidron D, Sira LB, Lerman-Sagie T. Fetal cytomegalovirus infection of the brain: the spectrum of sonographic findings. AJNR Am J Neuroradiol. 2003;24:28-32. [PubMed] |
| 8. | Dogan Y, Yuksel A, Kalelioglu IH, Has R, Tatli B, Yildirim A. Intracranial ultrasound abnormalities and fetal cytomegalovirus infection: report of 8 cases and review of the literature. Fetal Diagn Ther. 2011;30:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Nicloux M, Peterman L, Parodi M, Magny JF. Outcome and management of newborns with congenital cytomegalovirus infection. Arch Pediatr. 2020;27:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Kim Y, Kim YM, Kim DR, Kim HG, Sung JH, Choi SJ, Oh SY, Kim YJ, Chang YS, Kim D, Kim JS, Moon IJ, Roh CR. The Multifaceted Clinical Characteristics of Congenital Cytomegalovirus Infection: From Pregnancy to Long-Term Outcomes. J Korean Med Sci. 2023;38:e249. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Blázquez-Gamero D, Galindo Izquierdo A, Del Rosal T, Baquero-Artigao F, Izquierdo Méndez N, Soriano-Ramos M, Rojo Conejo P, González-Tomé MI, García-Burguillo A, Pérez Pérez N, Sánchez V, Ramos-Amador JT, De la Calle M. Prevention and treatment of fetal cytomegalovirus infection with cytomegalovirus hyperimmune globulin: a multicenter study in Madrid. J Matern Fetal Neonatal Med. 2019;32:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, Vergani P, Todros T, Frusca T, Arossa A, Furione M, Rognoni V, Rizzo N, Gabrielli L, Klersy C, Gerna G; CHIP Study Group. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 332] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 13. | Leruez-Ville M, Ville Y. Is it time for routine prenatal serological screening for congenital cytomegalovirus? Prenat Diagn. 2020;40:1671-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Malinger G, Lev D, Lerman-Sagie T. Imaging of fetal cytomegalovirus infection. Fetal Diagn Ther. 2011;29:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Leruez-Ville M, Ren S, Magny JF, Jacquemard F, Couderc S, Garcia P, Maillotte AM, Benard M, Pinquier D, Minodier P, Astruc D, Patural H, Ugolin M, Parat S, Guillois B, Garenne A, Parodi M, Bussières L, Stirnemann J, Sonigo P, Millischer AE, Ville Y. Accuracy of prenatal ultrasound screening to identify fetuses infected by cytomegalovirus which will develop severe long-term sequelae. Ultrasound Obstet Gynecol. 2021;57:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Leibovitz Z, Shkolnik C, Haratz KK, Malinger G, Shapiro I, Lerman-Sagie T. Assessment of fetal midbrain and hindbrain in mid-sagittal cranial plane by three-dimensional multiplanar sonography. Part 1: comparison of new and established nomograms. Ultrasound Obstet Gynecol. 2014;44:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Volpe N, Dall'Asta A, Di Pasquo E, Frusca T, Ghi T. First-trimester fetal neurosonography: technique and diagnostic potential. Ultrasound Obstet Gynecol. 2021;57:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Bronshtein M, Zimmer EZ, Auslander R, Blazer S. Isolated hyperechogenic foci in the fetal thalamus in early pregnancy. Ultrasound Obstet Gynecol. 2001;17:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Di Mascio D, Rizzo G, Khalil A, D'Antonio F; ENSO Working Group. Role of fetal magnetic resonance imaging in fetuses with congenital cytomegalovirus infection: multicenter study. Ultrasound Obstet Gynecol. 2023;61:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Prayer D, Malinger G, De Catte L, De Keersmaecker B, Gonçalves LF, Kasprian G, Laifer-Narin S, Lee W, Millischer AE, Platt L, Prayer F, Pugash D, Salomon LJ, Sanz Cortes M, Stuhr F, Timor-Tritsch IE, Tutschek B, Twickler D, Raine-Fenning N; ISUOG Clinical Standards Committee. ISUOG Practice Guidelines (updated): performance of fetal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2023;61:278-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |